Tantalum carbide
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Tantalum carbide
|
|
| Other names
Tantalum(IV) carbide
|
|
| Identifiers | |
|
12070-06-3 12070-07-04 (TaC0.5) |
|
| ECHA InfoCard | 100.031.914 |
| EC Number | 235-118-3 |
| Properties | |
| TaC | |
| Molar mass | 192.96 g/mol |
| Appearance | Brown-gray powder |
| Odor | Odorless |
| Density | 14.3–14.65 g/cm3 (TaC) 15.1 g/cm3 (TaC0.5) |
| Melting point | 3,850–3,880 °C (6,960–7,020 °F; 4,120–4,150 K) (TaC) 3,327 °C (6,021 °F; 3,600 K) (TaC0.5) |
| Boiling point | 4,780–5,470 °C (8,640–9,880 °F; 5,050–5,740 K) (TaC) |
| Insoluble | |
| Solubility | Soluble in HF-HNO3 mixture |
| Thermal conductivity | 21 W/m·K |
| Thermochemistry | |
| 36.71 J/mol·K | |
|
Std molar
entropy (S |
42.29 J/mol·K |
|
Std enthalpy of
formation (ΔfH |
−144.1 kJ/mol |
| Related compounds | |
|
Related refractory ceramic materials
|
Zirconium nitride Niobium carbide Zirconium carbide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Tantalum carbides form a family of binary chemical compounds of tantalum and carbon with the empirical formula TaCx, where x usually varies between 0.4 and 1. They are extremely hard, brittle, refractory ceramic materials with metallic electrical conductivity. They appear as brown-gray powders, which are usually processed by sintering. Being important cermet materials, tantalum carbides are commercially used in tool bits for cutting applications and are sometimes added to tungsten carbide alloys. The melting points of tantalum carbides peak at about 3880 °C depending on the purity and measurement conditions; this value is among the highest for binary compounds. Only tantalum hafnium carbide may have a slightly higher melting point of about 3942 °C, whereas the melting point of hafnium carbide is comparable to that of TaC.
TaCx powders of desired composition are prepared by heating a mixture of tantalum and graphite powders in vacuum or inert-gas atmosphere (argon). The heating is performed at temperature of about 2000 °C using a furnace or an arc-melting setup. An alternative technique is reduction of tantalum pentoxide by carbon in vacuum or hydrogen atmosphere at a temperature of 1500–1700 °C. This method was used to obtain tantalum carbide in 1876, but it lacks control over the stoichiometry of the product. Production of TaC directly from the elements has been reported through self-propagating high-temperature synthesis.
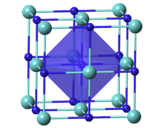
TaCx compounds have a cubic (rock-salt) crystal structure for x = 0.7–1.0; the lattice parameter increases with x. TaC0.5 has two major crystalline forms. The more stable one has an anti-cadmium iodide-type trigonal structure, which transforms upon heating to about 2000 °C into a hexagonal lattice with no long-range order for the carbon atoms.
...
Wikipedia
