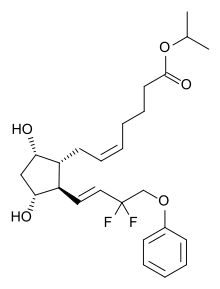
Tafluprost
 |
|
| Clinical data | |
|---|---|
| Trade names | Saflutan, Taflotan, Tapros, Zioptan |
| AHFS/Drugs.com | Multum Consumer Information |
| Pregnancy category |
|
| Routes of administration |
Topical (eye drops) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Activation by ester hydrolysis, deactivation by beta oxidation |
| Onset of action | 2–4 hrs |
| Duration of action | ≥ 24 hrs |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.207.745 |
| Chemical and physical data | |
| Formula | C25H34F2O5 |
| Molar mass | 452.531266 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Tafluprost (trade names Taflotan by Santen Pharmaceutical and Zioptan by Merck in the US) is a prostaglandin analogue. It is used topically (as eye drops) to control the progression of open-angle glaucoma and in the management of ocular hypertension, alone or in combination with other medication. It reduces intraocular pressure by increasing the outflow of aqueous fluid from the eyes.
The most common side effect is conjunctival hyperemia, which occurs in 4 to 20% of patients. Less common side effects include stinging of the eyes, headache, and respiratory infections. Rare side effects are dyspnoea (breathing difficulties), worsening of asthma, and macular oedema.
Nonsteroidal anti-inflammatory drugs (NSAIDs) can either reduce or increase the effect of tafluprost.Timolol eye drops, a common kind of glaucoma medication, does not negatively interact with this drug.
No interactions with systemic (for example, oral) drugs are expected because tafluprost does not reach relevant concentrations in the bloodstream.
Tafluprost is a prodrug of the active substance, tafluprost acid, a structural and functional analogue of prostaglandin F2α (PGF2α). Tafluprost acid is a selective agonist at the prostaglandin F receptor, increasing outflow of aqueous fluid from the eyes and thus lowering intraocular pressure.
...
Wikipedia
