Synaptobrevin
| Synaptobrevin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
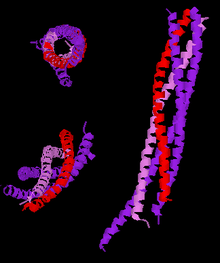
Three different views of the high resolution structure of a truncated neuronal SNARE complex. Legend: synaptobrevin-2 (red), Syntaxin-1 (pink), SNAP-25 (purple).
|
|||||||||
| Identifiers | |||||||||
| Symbol | Synaptobrevin | ||||||||
| Pfam | PF00957 | ||||||||
| InterPro | IPR001388 | ||||||||
| PROSITE | PDOC00368 | ||||||||
| SCOP | 1sfc | ||||||||
| SUPERFAMILY | 1sfc | ||||||||
| OPM superfamily | 218 | ||||||||
| OPM protein | 4wy4 | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
Synaptobrevins (synaptobrevin isotypes 1-2) are small integral membrane proteins of secretory vesicles with molecular weight of 18 kilodalton (kDa) that are part of the vesicle-associated membrane protein (VAMP) family.
Synaptobrevin is one of the SNARE proteins involved in formation of the SNARE complexes.
Out of four α-helices of the core SNARE complex one is contributed by synaptobrevin, one by syntaxin, and two by SNAP-25 (in neurons).
SNARE proteins are the key components of the molecular machinery that drives fusion of membranes in exocytosis. Their function however is subject to fine-tuning by various regulatory proteins collectively referred to as SNARE masters.
In the Q/R nomenclature for organizing SNARE proteins, VAMP/synaptobrevin family members are classified as R-SNAREs, so named for the presence of an arginine at a specific location within the primary sequence of the protein (as opposed to the SNAREs of the target membrane, which contain a glutamine and are so named Q-SNAREs). Synaptobrevin is classified as a V-SNARE in the V/T nomenclature, an alternative classification scheme in which SNAREs are classified as V-SNAREs and T-SNAREs for their localization to vesicles and target membranes, respectively.
Synaptobrevin is degraded by tetanospasmin, a protein derived from the bacterium Clostridium tetani, which causes tetanus. A related bacterium, Clostridium botulinum, produces botulinum toxin that also hydrolyzes synaptobrevin.
...
Wikipedia
