Sulfides
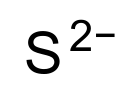 |
|
| Names | |
|---|---|
|
Systematic IUPAC name
Sulfanediide (substitutive)
Sulfide(2−) (additive) |
|
| Identifiers | |
|
18496-25-8 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15138 |
| ChemSpider |
27079 |
| PubChem | 29109 |
|
|
|
|
| Properties | |
| S2− |
|
| Molar mass | 32.06 g·mol−1 |
| Related compounds | |
|
Other anions
|
Telluride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Sulfide (systematically named sulfanediide, and sulfide(2−)) is an inorganic anion of sulfur with the chemical formula S2−. It contributes no color to sulfide salts. As it is classified as a strong base, even dilute solutions of salts such as sodium sulfide (Na2S) are corrosive and can attack the skin. Sulfide is the simplest sulfur anion.
The systematic names sulfanediide and sulfide(2−), valid IUPAC names, are determined according to the substitutive and additive nomenclatures, respectively. However, the name sulfide is also used in compositional IUPAC nomenclature which does not take the nature of bonding involved. Examples of such naming are selenium disulfide and titanium sulfide, which contains no sulfide ions whatsoever.
Sulfide is also used non-systematically, to describe compounds which release hydrogen sulfide upon acidification, or a compound that otherwise incorporates sulfur in some form, such as dimethyl sulfide. "Hydrogen sulfide" is itself an example of a non-systematic name of this nature. However, it is also a trivial name, and the preferred IUPAC name for sulfane.
Sulfide does not exist in appreciable concentrations even in highly alkaline water, being undetectable at pH < ~15 (8 M NaOH).
The sulfide anion can assimilate a proton by recombination:
Because of this capture of a proton (H+), sulfide has basic character. In aqueous solution, it has a pKb value of less than 0. Its conjugate acid is bisulfide (SH−). In aqueous solution, most sulfide ions are neutralized.
Upon treatment with a standard acid, sulfide converts to hydrogen sulfide (H2S) and a metal salt. Oxidation of sulfide gives sulfur or sulfate. Metal sulfides react with nonmetals including iodine, bromine, and chlorine forming sulfur and metal salts.
...
Wikipedia
