Stork quinine total synthesis
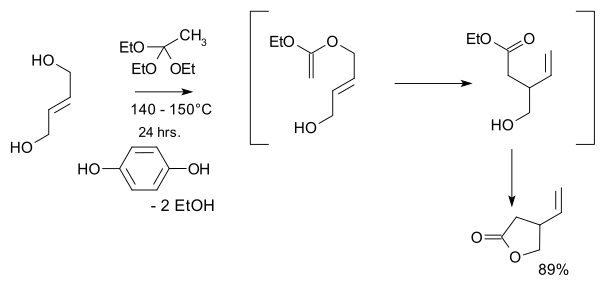
In total synthesis, the quinine total synthesis describes the efforts in synthesis of quinine over a 150-year period. The development of synthetic quinine is considered a milestone in organic chemistry although it has never been produced industrially as a substitute for natural occurring quinine. The subject has also been attended with some controversy: Gilbert Stork published the first stereoselective total synthesis of quinine in 2001, meanwhile shedding doubt on the earlier claim by Robert Burns Woodward and William Doering in 1944, claiming that the final steps required to convert their last synthetic intermediate, quinotoxine, into quinine would not have worked had Woodward and Doering attempted to perform the experiment. A 2001 editorial published in Chemical & Engineering News sided with Stork, but the controversy was eventually laid to rest once and for all when Williams and coworkers successfully repeated Woodward's proposed conversion of quinotoxine to quinine in 2007.
The aromatic component of the quinine molecule is a quinoline with a methoxy substituent. The amine component has a quinuclidine skeleton and the methylene bridge in between the two components has a hydroxyl group. The substituent at the carbon-3 position is a vinyl group. The molecule is optically active with five stereogenic centers (the N1 and C4 constituting a single asymmetric unit), making synthesis potentially difficult because it is one of 16 stereoisomers.
...
Wikipedia