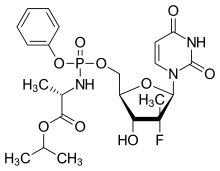
Sofosbuvir
 |
|
| Clinical data | |
|---|---|
| Trade names | Sovaldi, Soforal, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
by mouth |
| ATC code | J05AX15 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 92% |
| Protein binding | 61–65% |
| Metabolism | Quickly activated to triphosphate |
| Biological half-life | 0.4 hrs (sofosbuvir) 27 hrs (active metabolite GS-331007) |
| Excretion | 80% urine, 14% feces (mostly as GS-331007) |
| Identifiers | |
|
|
| Synonyms | PSI-7977; GS-7977 |
| CAS Number | 1190307-88-0 |
| PubChem (CID) | 45375808 |
| IUPHAR/BPS | 7368 |
| DrugBank | DB08934 |
| ChemSpider | 26286922 |
| UNII |
WJ6CA3ZU8B |
| KEGG | D10366 |
| ChEBI |
CHEBI:85083 |
| ChEMBL | CHEMBL1259059 |
| ECHA InfoCard | 100.224.393 |
| Chemical and physical data | |
| Formula | C22H29FN3O9P |
| Molar mass | 529.453 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used for the treatment of hepatitis C. It is only recommended with some combination of ribavirin, peginterferon-alfa, simeprevir, ledipasvir, or daclatasvir. Cure rates are 30 to 97% depending on the type of hepatitis C virus involved. Safety during pregnancy is unclear; while, some of the medications used in combination may result in harm to the baby. It is taken by mouth.
Common side effects include feeling tired, headache, nausea, and trouble sleeping. Side effects are generally more common in interferon-containing regimens. Sofosbuvir may reactivate hepatitis B in those who have been previously infected. In combination with ledipasvir, daclatasvir or simeprevir it it is not recommended with amiodarone due to the risk of an abnormally slow heartbeat. Sofosbuvir is in the nucleotide analog family of medication and works by blocking the hepatitis C NS5B protein.
Sofosbuvir was discovered in 2007 and approved for medical use in the United States in 2013. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. As of 2016 a 12-week course of treatment costs about $84,000 in the United States, $53,000 in the United Kingdom, $45,000 in Canada, and $483 in India. Over 60,000 people were treated with sofosbuvir in its first 30 weeks being sold in the United States.
Sofosbuvir is used for the treatment of chronic hepatitis C, genotypes 1, 2, 3, 4, 5, and 6, usually in combination with other medications depending on the specific genotype. For the treatment of genotypes 1, 4, 5, and 6 hepatitis C infections, sofosbuvir is used in combination with the viral NS5A inhibitor ledipasvir. For the treatment of other genotypes, sofosbuvir is used in combination with weight-based ribavirin alone in genotype 2 HCV infections, and together with peginterferon in genotype 3 HCV infections.
...
Wikipedia
