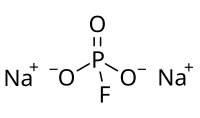
Sodium monofluorophosphate
 |
|
| Names | |
|---|---|
|
IUPAC name
Disodium phosphorofluoridate
|
|
| Other names
Sodium fluorophosphate, disodium monofluorophosphate
|
|
| Identifiers | |
|
10163-15-2 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:86431 |
| ChEMBL |
ChEMBL1200892 |
| ChemSpider |
22686 |
| ECHA InfoCard | 100.030.381 |
| EC Number | 233-433-0 |
| PubChem | 24266 |
| RTECS number | TE6130000 |
| UNII |
C810JCZ56Q |
|
|
|
|
| Properties | |
| Na2PFO3 | |
| Molar mass | 143.95 g/mol |
| Appearance | white powder |
| Melting point | 625 °C (1,157 °F; 898 K) |
| 25 g/100 mL | |
| Solubility | insoluble in ethanol, ether |
| Pharmacology | |
| A01AA02 (WHO) A12CD02 (WHO) | |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
502 mg/kg (rat, oral) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Sodium monofluorophosphate, commonly abbreviated MFP, is an inorganic compound with the chemical formula Na2PO3F. Typical for a salt, MFP is odourless, colourless, and water-soluble. This salt is an ingredient in some toothpastes.
MFP is best known as an ingredient in some toothpastes. It functions as a source of fluoride via the following hydrolysis reaction:
Fluoride protects tooth enamel from attack by bacteria that cause dental caries (cavities). Although developed by a chemist at Procter and Gamble, its use in toothpaste (Colgate toothpaste) was patented by Colgate-Palmolive, as Procter and Gamble was engaged in the marketing of Crest toothpaste (containing stannous fluoride, marketed as "Fluoristan"). In the early 1980s, Crest was reformulated to use MFP, under the trademark "Fluoristat"; today Crest toothpastes use sodium fluoride.
MFP is also used in some medications for the treatment of osteoporosis.
In 1991, sodium monofluorophosphate was found by Calgon to inhibit the dissolution of lead in drinking water when used in concentrations between 0.1 mg/L and 500 mg/L.
Tooth decay is caused by bacteria naturally present in one's mouth. These bacteria form a sticky, colorless soft film on the teeth called plaque. When foods containing carbohydrates (starches and sugars) are eaten, the bacteria that form plaque use the sugar as a form of energy. They also turn it into a glue-like substance that helps them stick to the surface of the tooth. The plaque produces acid, which attacks the enamel.
...
Wikipedia

