Secobarbital
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Seconal |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a682386 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 45-60% |
| Metabolism | Hepatic |
| Biological half-life | 15-40 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.000.894 |
| Chemical and physical data | |
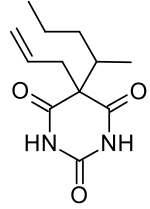
| Formula | C12H18N2O3 |
| Molar mass | 238.283 |
| 3D model (Jmol) | |
|
|
|
|
Secobarbital sodium (marketed by Eli Lilly and Company, and subsequently by other companies as described below, under the brand name Seconal) is a barbiturate derivative drug that was patented in 1934 in the US. It possesses anaesthetic, anticonvulsant, anxiolytic, sedative, and hypnotic properties. In the United Kingdom, it was known as quinalbarbitone. It is the most frequently used drug in physician-assisted suicide within the United States.
Secobarbital is indicated for:
Ranbaxy Pharmaceuticals, an India-based company now predominantly owned by the Japanese company Daiichi Sankyo, obtained the rights to market and to use the trade name "Seconal" from Eli Lilly in 1998, and did so until September 18, 2008. The actual manufacturer of Seconal subsequent to the time Eli Lilly manufactured the drug was Ohm Pharmaceuticals, a wholly owned subsidiary of Ranbaxy. The rights to market Seconal were then sold to Marathon Pharmaceuticals, which became the marketer and trade-name holder. In February 2015, Marathon sold the rights to Valeant Pharmaceuticals. However Seconal is still manufactured by Ohm. In the United States, Seconal is available only in 100 mg capsules, as a sodium salt. The salt is a white hygroscopic powder that is soluble in water and ethanol.
After the rights to Seconal were sold to Valeant Pharmaceuticals in 2015, Valeant immediately doubled the price in the United States from $1,500 per 100 capsules to $3,000. While generic versions of the drug used to exist after the Seconal patent expired in the early 1990s, there are currently no generics available on the US market, making Valeant the sole vendor of the drug in the US.
The sodium salt of secobarbital is classified separately from the free acid, as follows:
Possible side effects of secobarbital include:
...
Wikipedia
