Rylene dye
A rylene dye is a dye based on the rylene framework of naphthalene units linked in peri-positions. In homologues additional naphthalene units are added, forming compounds - or poly(peri-naphthalene)s - such as perylene, terrylene, quarterrylene.
Perylene dyes are useful for their intense visible light absorption, high stability, electron accepting ability, and unity quantum yields. Due to these properties, they are actively researched in academia for optoelectronic and photovoltaic devices, thermographic processes, energy-transfer cascades, light-emitting diodes, and near-infrared-absorbing systems. A bathochromic shift of about 100 nm is recorded per additional naphthalene unit.
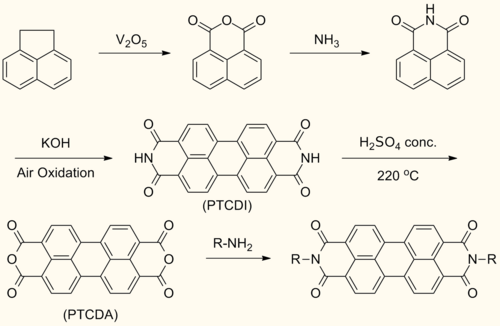
Perylenediimide (PDI) are synthesized by treating perylenetetracarboxylic dianhydride (PTCDA) with amines at high temperatures.
This reaction forms symmetrically N,N’- substituted PDIs. Solubilizing groups are often attached in this fashion. Although the solubility of the dianhydride is low, the solubility of some mono- and diimide derivatives are greatly improved.
Perylene diimide derivatives were initially developed as industrial dyes due to their excellent chemical, photo, thermal, and weather stability. Nowadays, perylene dyes are used predominantly in textile applications and as high-grade industrial paint.
Several perylene pigments have been developed for artist's paints :
Rylene dyes have been less popular as a fluorescent tag due to their low solubility in aqueous solutions. However, there has been much progress in tailoring rylene probes to this application. An advantage of using rylene dyes is that different functional groups can be placed in the imide structure and in the bay region. This has allowed polar carboxylic acid and sulfonic acid groups to be added to make rylene dyes more soluble. The imide structure allows for two different substituent, so a single reactive group can be attached to this position.
...
Wikipedia