Roundup
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
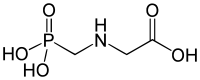
IUPAC name
N-(phosphonomethyl)glycine
|
|
| Other names
2-[(phosphonomethyl)amino]acetic acid
|
|
| Identifiers | |
|
|
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.012.726 |
| EC Number | 213-997-4 |
| KEGG | |
|
PubChem CID
|
|
| RTECS number | MC1075000 |
| UNII | |
|
|
|
|
| Properties | |
| C3H8NO5P | |
| Molar mass | 169.07 g·mol−1 |
| Appearance | white crystalline powder |
| Density | 1.704 (20 °C) |
| Melting point | 184.5 °C (364.1 °F; 457.6 K) |
| Boiling point | decomposes at 187 °C (369 °F; 460 K) |
| 1.01 g/100 mL (20 °C) | |
| log P | −2.8 |
| Acidity (pKa) | <2, 2.6, 5.6, 10.6 |
| Hazards | |
| Safety data sheet | InChem MSDS |
| GHS pictograms |
 
|
| GHS signal word | DANGER |
| H318, H411 | |
| P273, P280, P305+351+338, P310, P501 | |
|
EU classification (DSD)
|
Irritant (Xi) Dangerous for the environment (N) |
| R-phrases | R41, R51/53 |
| S-phrases | (S2), S26, S39, S61 |
| Flash point | Non-flammable |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Glyphosate (N-(phosphonomethyl)glycine) is a broad-spectrum systemic herbicide and crop desiccant. It is an organophosphorus compound, specifically a phosphonate. It is used to kill weeds, especially annual broadleaf weeds and grasses that compete with crops. It was discovered to be an herbicide by Monsanto chemist John E. Franz in 1970. Monsanto brought it to market in 1974 under the trade name Roundup, and Monsanto's last commercially relevant United States patent expired in 2000.
Farmers quickly adopted glyphosate, especially after Monsanto introduced glyphosate-resistant Roundup Ready crops, enabling farmers to kill weeds without killing their crops. In 2007, glyphosate was the most used herbicide in the United States' agricultural sector and the second-most used in home and garden (2,4-D being the most used), government and industry, and commerce. By 2016 there was a 100-fold increase from the late 1970s in the frequency of applications and volumes of glyphosate-based herbicides (GBHs) applied, partly in response to the unprecedented global emergence and spread of glyphosate-resistant weeds.
Glyphosate is absorbed through foliage, and minimally through roots, and transported to growing points. It inhibits a plant enzyme involved in the synthesis of three aromatic amino acids: tyrosine, tryptophan, and phenylalanine. Therefore, it is effective only on actively growing plants and is not effective as a pre-emergence herbicide. An increasing number of crops have been genetically engineered to be tolerant of glyphosate (e.g. Roundup Ready soybean, the first Roundup Ready crop, also created by Monsanto) which allows farmers to use glyphosate as a postemergence herbicide against weeds. The development of glyphosate resistance in weed species is emerging as a costly problem. While glyphosate and formulations such as Roundup have been approved by regulatory bodies worldwide, concerns about their effects on humans and the environment persist.
...
Wikipedia
