Rosiglitazone
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Avandia |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699023 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 99% |
| Protein binding | 99.8% |
| Metabolism | Hepatic (CYP2C8-mediated) |
| Biological half-life | 3–4 hours |
| Excretion | Renal (64%) and fecal (23%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
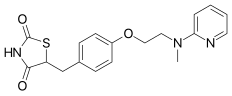
| Formula | C18H19N3O3S |
| Molar mass | 357.428 g/mol |
| 3D model (Jmol) | |
| Chirality | Racemic mixture |
|
|
|
|
|
|
|
Rosiglitazone (trade name Avandia, GlaxoSmithKline) is an antidiabetic drug in the thiazolidinedione class of drugs. It works as an insulin sensitizer, by binding to the PPAR receptors in fat cells and making the cells more responsive to insulin. It is marketed by the pharmaceutical company GlaxoSmithKline (GSK) as a stand-alone drug or for use in combination with metformin or with glimepiride. First released in 1999, annual sales peaked at approximately $2.5-billion in 2006; however, following a meta-analysis published in the New England Journal of Medicine in 2007 that linked the drug's use to an increased risk of heart attack, sales plummeted to just $9.5-million in 2012. The drug's patent expired in 2012.
Despite rosiglitazone's effectiveness at decreasing blood sugar in type 2 diabetes mellitus, its use decreased dramatically as studies showed apparent associations with increased risks of heart attacks and death. Adverse effects alleged to be caused by rosiglitazone were the subject of over 13,000 lawsuits against GSK; as of July 2010, GSK had agreed to settlements on more than 11,500 of these suits.
Some reviewers recommended rosiglitazone be taken off the market, but an FDA panel disagreed, and it remains available in the U.S. From November 2011 until November 2013, the federal government did not allow Avandia to be sold without a prescription from a certified doctor; moreover, patients were required to be informed of the risks associated with its use, and the drug had to be purchased by mail order through specified pharmacies. In November 2013, the FDA lifted its earlier restrictions on rosiglitazone after reviewing the results of the 2009 RECORD clinical trial (a six-year, open label randomized control trial), which failed to show heart attack risks associated with the drug.
...
Wikipedia
