Radioactive element
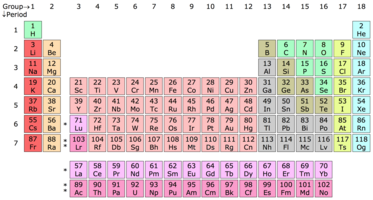 |
|
| Top: The periodic table of the chemical elements. Below: Examples of certain chemical elements. From left to right: hydrogen, barium, copper, uranium, bromine, and helium. |
A chemical element or element is a species of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up Earth, while oxygen is the most common element in the Earth's crust.
Chemical elements constitute all of the ordinary matter of the universe. However astronomical observations suggest that ordinary observable matter makes up only about 15% of the matter in the universe: the remainder is dark matter; the composition of this is unknown, but it is not composed of chemical elements. The two lightest elements, hydrogen and helium, were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus rarer than those that follow. Formation of elements with from 6 to 26 protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than 26 protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements as supernova remnants far into space, where they may become incorporated into planets when they are formed.
...
Wikipedia






