Quinine total synthesis
In total synthesis, the quinine total synthesis describes the efforts in synthesis of quinine over a 150-year period. The development of synthetic quinine is considered a milestone in organic chemistry although it has never been produced industrially as a substitute for natural occurring quinine. The subject has also been attended with some controversy: in 2001 Gilbert Stork published the first stereoselective quinine synthesis and he shed doubt (calling it a myth) on the earlier claim in 1944 by R.B. Woodward and William Doering on account that they had obtained not quinine but a precursor molecule. In 2001, an editorial in Chemical & Engineering News supported Storks claim but according to a critical 30 page review in this matter published in 2007 in Angewandte Chemie the Woodward/Doering claim is valid.
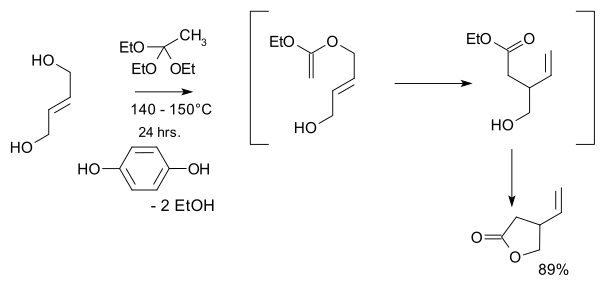
The aromatic component of the quinine molecule is a quinoline with a methoxy substituent. The amine component has a quinuclidine skeleton and the methylene bridge in between the two components has a hydroxyl group. The substituent at the carbon-3 position is a vinyl group. The molecule is optically active with five stereogenic centers (the N1 and C4 constituting a single asymmetric unit), making synthesis potentially difficult because it is one of 16 stereoisomers.
...
Wikipedia