Quetiapine
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /kwᵻˈtaɪ.əpiːn/ kwi-TY-ə-peen |
| Trade names | Seroquel |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698019 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | N05AH04 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | 83% |
| Metabolism | Hepatic via CYP3A4-catalysed sulfoxidation to its active metabolite norquetiapine (N-desalkylquetiapine) |
| Biological half-life | 7 hours (parent compound); 9–12 hours (active metabolite, norquetiapine) |
| Excretion | Renal (73%), faeces (20%) |
| Identifiers | |
|
|
| CAS Number |
111974-69-7 |
| PubChem (CID) | 5002 |
| IUPHAR/BPS | 50 |
| DrugBank |
DB01224 |
| ChemSpider |
4827 |
| UNII |
BGL0JSY5SI |
| KEGG |
D08456 |
| ChEBI |
CHEBI:8707 |
| ChEMBL |
CHEMBL716 |
| ECHA InfoCard | 100.131.193 |
| Chemical and physical data | |
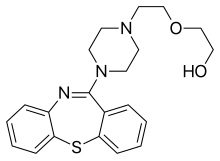
| Formula | C21H25N3O2S |
| Molar mass | 383.5099 g/mol |
| 3D model (Jmol) | Interactive image |
| Solubility in water | 3.29 mg/mL (20 °C) |
|
|
|
|
Quetiapine, marketed as Seroquel, is an atypical antipsychotic approved for the treatment of schizophrenia, bipolar disorder, and along with an antidepressant to treat major depressive disorder. It is also sometimes used as a sleep aid because of its sedating effect but this use is not recommended.
Annual sales are approximately $1.3 billion worldwide. Quetiapine was developed by AstraZeneca from 1992 to 1996. It was first approved by the FDA in 1997. There are now several generic versions.
Quetiapine is primarily used to treat schizophrenia or bipolar disorder.
A second Cochrane Review comparing quetiapine to typical antipsychotics concluded that quetiapine
There is tentative evidence of the benefit of quetiapine versus placebo in schizophrenia; however, definitive conclusions are not possible due to the high rate of attrition in trials (greater than 50%) and the lack of data on economic outcomes, social functioning, or quality of life.
It is debatable whether, as a class, typical or atypical antipsychotics are more effective. Both have equal drop-out and symptom relapse rates when typicals are used at low to moderate dosages. While quetiapine has lower rates of extrapyramidal side effects, there is greater sleepiness and rates of dry mouth.
A Cochrane Review comparing quetiapine to other atypical antipsychotic agents tentatively concluded that it may be less efficacious than olanzapine and risperidone; produce fewer movement related side effects than paliperidone, aripiprazole, ziprasidone, risperidone and olanzapine; and produce weight gain similar to risperidone, clozapine and aripiprazole.
In those with bipolar disorder, quetiapine is used to treat depressive episodes, acute manic episodes associated with bipolar I disorder (as either monotherapy or adjunct therapy to lithium, valproate or lamotrigine), and maintenance treatment of bipolar I disorder (as adjunct therapy to lithium or divalproex).
...
Wikipedia
