Quaternium-15
 |
|
| Names | |
|---|---|
|
IUPAC name
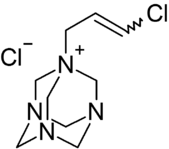
1-(3-Chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride
|
|
Other names
|
|
| Identifiers | |
|
|
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.021.641 |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C9H16Cl2N4 | |
| Molar mass | 251.16 g·mol−1 |
| Hazards | |
| Safety data sheet | Sigma Aldrich |
| GHS pictograms |
   
|
| H228, H302, H315, H317, H361, H411 | |
| P210, P273, P280 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Quaternium-15 (systematic name: hexamethylenetetramine chloroallyl chloride) is a quaternary ammonium salt used as a surfactant and preservative in many cosmetics and industrial substances. It is an anti-microbial agent by virtue of being a formaldehyde releaser, however this can also cause contact dermatitis, a symptom of an allergic reaction, especially in those with sensitive skin.
It can be found under a variety of names, most commonly those of the Dow Chemical Company: Dowicil 200 (cis isomer only), Dowicil 75 and Dowicil 100 (both a mix of cis and trans isomers).
Quaternium-15 can be prepared by reacting hexamethylenetetramine with 1,3-dichloropropene to produce the product as a mixture of cis and trans isomers.
The isolated cis-compound is used primarily in cosmetic applications, with a maximum permitted concentration in the EU of 0.2%. The mixed product (cis- and trans-) is used in a wider range of formulations such as: emulsifiable metal-cutting fluids; latex and emulsion paints; liquid floor polishes and floor waxes; glues and adhesives.
Quaternium-15 is an allergen, and can cause contact dermatitis in susceptible individuals. Many of those with an allergy to quaternium-15 are also allergic to formaldehyde. At low pHs it would be expected to release significant amounts of formaldehyde due to acid hydrolysis via the Delepine reaction.
Allergic sensitivity to quaternium-15 can be detected using a patch test. It is the single most often found cause of allergic contact dermatitis of the hands (16.5% in 959 cases). In 2005–06, it was the fourth-most-prevalent allergen in patch tests (10.3%).
Some consumer cosmetics contain quaternium-15 for its antimicrobial properties. The American Cancer Society states that although quaternium-15 releases formaldehyde, a known carcinogen in laboratory test animals at relatively high doses, because the amount of formaldehyde released from these products is low, it is unclear that avoiding quaternium-15 in cosmetics provides any health benefits. Even so, Johnson & Johnson announced plans to phase out its use of quaternium-15 in cosmetic products by 2015 in response to consumer pressure.
...
Wikipedia
