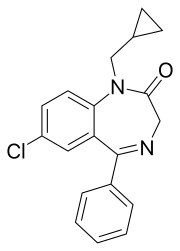
Prazepam
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601036 |
| Routes of administration |
Oral |
| ATC code | N05BA11 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Biological half-life | 36–200 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| Synonyms | 9-chloro-2-(cyclopropylmethyl)-6-phenyl-2,5-diazabicyclo[5.4.0]undeca-5,8,10,12-tetraen- 3-one |
| CAS Number |
2955-38-6 |
| PubChem (CID) | 4890 |
| IUPHAR/BPS | 7275 |
| DrugBank |
DB01588 |
| ChemSpider |
4721 |
| UNII |
Q30VCC064M |
| KEGG |
D00470 |
| ChEMBL |
CHEMBL969 |
| ECHA InfoCard | 100.019.069 |
| Chemical and physical data | |
| Formula | C19H17ClN2O |
| Molar mass | 324.8 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Prazepam is a benzodiazepine derivative drug developed by Warner-Lambert in the 1960s. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. Prazepam is a prodrug for desmethyldiazepam which is responsible for the therapeutic effects of prazepam.
Prazepam is indicated for the short-term treatment of anxiety. After short-term therapy, the dose is usually gradually tapered-off to reduce or avoid any withdrawal or rebound effects.Desmethyldiazepam, an active metabolite, has a very long half-life of 29 to 224 hours, which contributes to the therapeutic effects of prazepam.
Side effects of prazepam are less profound than with other benzodiazepines. Excessive drowsiness and with longer-term use, drug dependence, are the most common side effects of prazepam. Side effects such as fatigue or "feeling spacey" can also occur but less commonly than with other benzodiazepines. Other side effects include feebleness, clumsiness or lethargic, clouded thinking and mental slowness.
Tolerance and dependence can develop with long-term use of prazepam, and upon cessation or reduction in dosage, then a benzodiazepine withdrawal syndrome may occur with symptoms such as tremulousness, dysphoria, psychomotor agitation, tachycardia and sweating. In severe cases, hallucinations, psychosis and seizures can occur. Withdrawal-related psychosis is generally unresponsive to antipsychotic mediations. The risk and severity of the withdrawal syndrome increases the higher the dose and the longer prazepam is taken for. Tolerance, dependence and withdrawal problems may be less severe than with other benzodiazepines, such as diazepam. It may be because tolerance is slower to develop with prazepam than with other benzodiazepines. Abrupt or over-rapid discontinuation of prazepam after long-term use, even at low dosage, may result in a protracted withdrawal syndrome.
...
Wikipedia
