Pravastatin
 |
|
| Clinical data | |
|---|---|
| Trade names | Pravachol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692025 |
| Pregnancy category |
|
| Routes of administration |
oral |
| ATC code | C10AA03 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 18% |
| Protein binding | 50% |
| Metabolism | Hepatic (minimal) |
| Biological half-life | 1-3 hours |
| Identifiers | |
|
|
| CAS Number |
81093-37-0 |
| PubChem (CID) | 54687 |
| IUPHAR/BPS | 2953 |
| DrugBank |
DB00175 |
| ChemSpider |
49398 |
| UNII |
KXO2KT9N0G |
| ChEBI |
CHEBI:63618 |
| ChEMBL |
CHEMBL1144 |
| ECHA InfoCard | 100.216.225 |
| Chemical and physical data | |
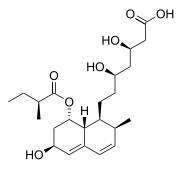
| Formula | C23H36O7 |
| Molar mass | 424.528 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Pravastatin (marketed as Pravachol or Selektine) is a member of the drug class of statins, used in combination with diet, exercise, and weight loss for lowering cholesterol and preventing cardiovascular disease.
Pravastatin is primarily used for the treatment of dyslipidemia and the prevention of cardiovascular disease. It is recommended to be used only after other measures, such as diet, exercise, and weight reduction, have not improved cholesterol levels.
The evidence for the use of pravastatin is generally weaker than for other statins. The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT), failed to demonstrate a difference in all-cause mortality or nonfatal myocardial infarction/fatal coronary heart disease rates between patients receiving pravastatin 40 mg daily (a common starting dose) and those receiving usual care.
Pravastatin has undergone over 112,000 patient-years of double-blind, randomized trials using the 40-mg, once-daily dose and placebos. These trials indicate pravastatin is well tolerated and displays few noncardiovascular abnormalities in patients. However, side effects may occur. A doctor should be consulted if symptoms such as heartburn or headache are severe and do not go away.
These uncommon side effects should be promptly reported to the prescribing doctor or an emergency medical service:
These symptoms should be reported to the prescribing doctor if they persist or increase in severity:
Contraindications, conditions that warrant withholding treatment with pravastatin, include pregnancy and breastfeeding. Taking pravastatin while pregnant could lead to birth defects. While the amount of pravastatin ingested by an infant from breastfeeding is low, patients breastfeeding should not take pravastatin due to potential effects on the infant's lipid metabolism.
Medications that should not be taken with pravastatin include, but are not limited to:
Pravastatin is cleared by the kidney, giving it a distinct advantage over other statins when a potential for drug interactions using the hepatic pathway exists.
Pravastatin acts as a lipoprotein-lowering drug through two pathways. In the major pathway, pravastatin inhibits the function of hydroxymethylglutaryl-CoA (HMG-CoA) reductase. As a reversible competitive inhibitor, pravastatin sterically hinders the action of HMG-CoA reductase by occupying the active site of the enzyme. Taking place primarily in the liver, this enzyme is responsible for the conversion of HMG-CoA to mevalonate in the rate-limiting step of the biosynthetic pathway for cholesterol. Pravastatin also inhibits the synthesis of very-low-density lipoproteins, which are the precursor to low-density lipoproteins (LDL). These reductions increase the number of cellular LDL receptors, thus LDL uptake increases, removing it from the bloodstream. Overall, the result is a reduction in circulating cholesterol and LDL. A minor reduction in triglycerides and an increase in high-density lipoproteins (HDL) are common.
...
Wikipedia
