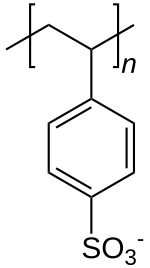
Polystyrene sulfonate
 |
|
| Clinical data | |
|---|---|
| Trade names |
Sodium salt: Kayexalate, Kionex, Resonium A Calcium salt: Calcium Resonium, Sorbisterit, Resikali |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682108 |
| Pregnancy category |
|
| Routes of administration |
Oral, retention enema |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | None |
| Metabolism | None |
| Excretion | Faeces (100%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider |
|
| KEGG | |
| ECHA InfoCard | 100.167.553 |
| Chemical and physical data | |
| Formula | [C8H7SO3−]n |
|
|
|
Polystyrene sulfonates are polymers derived from polystyrene but containing sulfonate functional groups. They are widely used as ion-exchange resins to remove ions such as potassium, calcium and sodium from solutions in technical or medical applications.
Linear ionic polymers are generally water-soluble, whereas cross-linked materials (called resins) do not dissolve in water. These polymers are classified as polysalts and ionomers.
Polystyrene sulfonate is usually supplied in the sodium and calcium form. It is used as a potassium binder in acute and chronic kidney disease for people with hyperkalemia (abnormal high blood serum potassium levels). It however is unclear if it works and there is concern about possible side effects when it is mixed with sorbitol.
Polystyrene sulfonates are given by mouth (with a meal) or rectally, by retention enema.
Under the name tolevamer, a polystyrene sulfonate was investigated by Genzyme as a toxin binding agent for the treatment of Clostridium difficile associated diarrhoea (CDAD), but never marketed.
The drug is contraindicated in patients with obstructive bowel disease and in newborn children with reduced gut motility.
Intestinal disturbances are common, including loss of appetite, nausea, vomiting, and constipation. In rare cases, it has been associated with colonic necrosis. Changes in electrolyte blood levels may occur such as hypermagnesemia, hypercalcemia, and hypokalemia.
...
Wikipedia
