Polyphthalamide
Polyphthalamide (aka. PPA, High Performance Polyamide) is a subset of thermoplastic synthetic resins in the polyamide (nylon) family defined as when 55% or more moles of the carboxylic acid portion of the repeating unit in the polymer chain is composed of a combination of terephthalic (TPA) and isophthalic (IPA) acids. The substitution of aliphatic diacids by aromatic diacids in the polymer backbone increases the melting point, glass transition temperature, chemical resistance and stiffness.
PPA based resins are molded into parts to replace metals in applications requiring high temperature resistance such as automotive powertrain components, the housing for high temperature electrical connectors and many other uses.
The diamines in PPAs are aliphatic. PA6T homopolymer melts at 371 °C, which renders it intractable. To make usable polymers, it is necessary to lower the melting point, which can be achieved practically using either a longer diamine (with 9-12 carbon atoms) or by copolymerizing 6T.
Three copolymers have found commercial success: PA 6T/66, PA 6T/"DT" and PA6T/6I.
 Polyphthalamide with 6T-Segment
Polyphthalamide with 6T-Segment
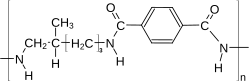 Polyphthalamide with DT-Segment
Polyphthalamide with DT-Segment
If more than 55% of the acid part of a PPA is made out of IPA, then the copolymer is amorphous.
Molar masses for PPAs made with direct polycondensation techniques range between 12,000 and 16,000 g/mol.
Compared to aliphatic polyamides, PPAs offer improved
The glass transition temperature of PPA increases as the amount of TPA increases. If more than 55% of the acid part of a PPA is made out of IPA, then the copolymer is amorphous. The properties of semicrystalline polymers v amorphous polymers are described elsewhere in detail. Briefly, crystallinity helps with chemical resistance and mechanical properties above the glass transition temperature (but below the melting point). Amorphous polymers are good in warpage and transparency.
...
Wikipedia
