Poly(dimethylsiloxane)
 |
|
| Names | |
|---|---|
|
IUPAC name
poly(dimethylsiloxane)
|
|
| Other names
PDMS
dimethicone dimethylpolysiloxane E900 |
|
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.126.442 |
| E number | E900 (glazing agents, ...) |
| UNII | |
| Properties | |
| (C2H6OSi)n | |
| Density | 965 kg m−3 |
| Melting point | N/A (vitrifies) |
| Boiling point | N/A (vitrifies) |
| Pharmacology | |
| P03AX05 (WHO) | |
| Hazards | |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Polydimethylsiloxane (PDMS) belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer, and is particularly known for its unusual rheological (or flow) properties. PDMS is optically clear, and, in general, inert, non-toxic, and non-flammable. It is also called dimethicone and is one of several types of silicone oil (polymerized siloxane). Its applications range from contact lenses and medical devices to elastomers; it is also present in shampoos (as dimethicone makes hair shiny and slippery), food (antifoaming agent), caulking, lubricants and heat-resistant tiles.
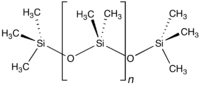
The chemical formula for PDMS is CH3[Si(CH3)2O]nSi(CH3)3, where n is the number of repeating monomer [SiO(CH3)2] units. Industrial synthesis can begin from dimethyldichlorosilane and water by the following net reaction:
The polymerization reaction evolves hydrochloric acid. For medical and domestic applications, a process was developed in which the chlorine atoms in the silane precursor were replaced with acetate groups. In this case, the polymerization produces acetic acid, which is less chemically aggressive than HCl. As a side-effect, the curing process is also much slower in this case. The acetate is used in consumer applications, such as silicone caulk and adhesives.
...
Wikipedia

