Plutonium(III) chloride
 |
|
| Names | |
|---|---|
|
IUPAC name
Plutonium(III) chloride
|
|
| Other names
Plutonium trichloride
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
|
|
|
|
| Properties | |
| Cl3Pu | |
| Molar mass | 350.322 g/mol |
| Appearance | Green solid |
| Density | 5.71 g/cm3, solid |
| Melting point | 767 °C (1,413 °F; 1,040 K) |
| Boiling point | 1,767 °C (3,213 °F; 2,040 K) |
| Hazards | |
|
EU classification (DSD) (outdated)
|
not listed |
| Related compounds | |
|
Other anions
|
PuCl4, PuBr3, SmCl3 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Plutonium(III) chloride is the chemical compound with the formula PuCl3. It can be prepared by dissolving the metal in hydrochloric acid.
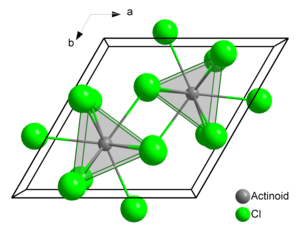
Plutonium atoms in crystalline PuCl3 are 9 coordinate, and the structure is tricapped trigonal prismatic.
As with all plutonium compounds, it is subject to control under the Nuclear Non-Proliferation Treaty. Due to the radioactivity of plutonium, all of its compounds, PuCl3 included, are warm to the touch. Such contact is not recommended, since touching the material may result in serious injury.
...
Wikipedia
