Plerixafor
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Mozobil |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a609018 |
| Pregnancy category |
|
| Routes of administration |
Subcutaneous injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Up to 58% |
| Metabolism | None |
| Biological half-life | 3–5 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| Synonyms | JM 3100, AMD3100 |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
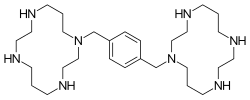
| Formula | C28H54N8 |
| Molar mass | 502.782 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Plerixafor (INN and USAN, trade name Mozobil) is an immunostimulant used to mobilize hematopoietic stem cells in cancer patients into the bloodstream. The stem cells are then extracted from the blood and transplanted back to the patient. The drug was developed by AnorMED which was subsequently bought by Genzyme.
Peripheral blood stem cell mobilization, which is important as a source of hematopoietic stem cells for transplantation, is generally performed using granulocyte colony-stimulating factor (G-CSF), but is ineffective in around 15 to 20% of patients. Combination of G-CSF with plerixafor increases the percentage of persons that respond to the therapy and produce enough stem cells for transplantation. The drug is approved for patients with lymphoma and multiple myeloma.
Studies in pregnant animals have shown teratogenic effects. Plerixafor is therefore contraindicated in pregnant women except in critical cases. Fertile women are required to use contraception. It is not known whether the drug is secreted into the breast milk. Breast feeding should be discontinued during therapy.
Nausea, diarrhea and local reactions were observed in over 10% of patients. Other problems with digestion and general symptoms like dizziness, headache, and muscular pain are also relatively common; they were found in more than 1% of patients. Allergies occur in less than 1% of cases. Most adverse effects in clinical trials were mild and transient.
The European Medicines Agency has listed a number of safety concerns to be evaluated on a post-marketing basis, most notably the theoretical possibilities of spleen rupture and tumor cell mobilisation. The first concern has been raised because splenomegaly was observed in animal studies, and G-CSF can cause spleen rupture in rare cases. Mobilisation of tumor cells has occurred in patients with leukaemia treated with plerixafor.
...
Wikipedia
