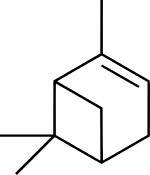
Pinene
 |
|
| Names | |
|---|---|
|
IUPAC names
(1S,5S)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene
(1S,5S)-6,6-dimethyl-2-methylenebicyclo[3.1.1]heptane |
|
| Identifiers | |
|
80-56-8 (unspecified) 7785-70-8 (1R-α) 7785-26-4 (1S-α) 2437-95-8 ((±)-α) 18172-67-3 (β) |
|
| ECHA InfoCard | 100.029.170 |
| Properties | |
| C10H16 | |
| Molar mass | 136.24 g/mol |
| Appearance | Liquid |
| Density | 0,86 g·cm−3 (alpha, 15 °C) |
| Melting point | −62 to −55 °C (−80 to −67 °F; 211 to 218 K) (alpha) |
| Boiling point | 155 to 156 °C (311 to 313 °F; 428 to 429 K) (alpha) |
| Practically insoluble in water | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Pinene (C10H16) is a bicyclic monoterpene chemical compound. There are two structural isomers of pinene found in nature: α-pinene and β-pinene. As the name suggests, both forms are important constituents of pine resin; they are also found in the resins of many other conifers, as well as in non-coniferous plants such as camphorweed (Heterotheca) and big sagebrush (Artemisia tridentata). Both isomers are used by many insects in their chemical communication system. The two isomers of pinene constitute the major component of turpentine.
α-Pinene and β-pinene are both produced from geranyl pyrophosphate, via cyclisation of linaloyl pyrophosphate followed by loss of a proton from the carbocation equivalent.
Alpha-pinene is the most widely encountered terpenoid in nature and is highly repellant to insects.
Alpha-pinene appears in conifers and numerous other plants. Pinene is a major component of the essential oils of Sideritis spp. (ironwort) and Salvia spp. (sage).Cannabis also contains alpha-pinene. Resin from Pistacia terebinthus (commonly known as terebinth or turpentine tree) is rich in pinene. Pine nuts produced by pine trees contain pinene.
Makrut lime fruit peel contains an essential oil comparable to lime fruit peel oil; its main components are limonene and β-pinene.
...
Wikipedia
