Phosphorus oxychloride
 |
|||
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
IUPAC names
Phosphoryl trichloride
Phosphorus trichloride oxide |
|||
| Other names
Phosphorus oxychloride
Phosphoric trichloride Trichlorophosphate |
|||
| Identifiers | |||
|
10025-87-3 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:30336 |
||
| ChemSpider |
23198 |
||
| ECHA InfoCard | 100.030.030 | ||
| EC Number | 233-046-7 | ||
| PubChem | 24813 | ||
| RTECS number | TH4897000 | ||
| UNII |
9XM78OL22K |
||
| UN number | 1810 | ||
|
|||
|
|||
| Properties | |||
| POCl3 | |||
| Molar mass | 153.33 g/mol | ||
| Appearance | Clear, colourless liquid, fumes in moist air | ||
| Odor | pungent and musty | ||
| Density | 1.645 g/cm3, liquid | ||
| Melting point | 1.25 °C (34.25 °F; 274.40 K) | ||
| Boiling point | 105.8 °C (222.4 °F; 378.9 K) | ||
| Reacts | |||
| Vapor pressure | 40 mmHg (27°C) | ||
| Structure | |||
| tetrahedral | |||
| 2.54 D | |||
| Hazards | |||
| Safety data sheet |
See: data page ICSC 0190 |
||
|
EU classification (DSD)
|
Very toxic (T+) Harmful (Xn) Corrosive (C) |
||
| R-phrases | R14, R22, R26, R35, R48/23 | ||
| S-phrases | (S1/2), S7/8, S26, S36/37/39, S45 | ||
| NFPA 704 | |||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
none | ||
|
REL (Recommended)
|
TWA 0.1 ppm (0.6 mg/m3) ST 0.5 ppm (3 mg/m3) | ||
|
IDLH (Immediate danger)
|
N.D. | ||
| Related compounds | |||
|
Related compounds
|
Thiophosphoryl chloride Phosphorus oxybromide Phosphorus trichloride Phosphorus pentachloride |
||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and choking fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate.
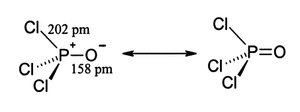
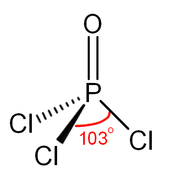
Like phosphate, phosphoryl chloride is tetrahedral in shape. It features three P-Cl bonds and one strong P=O double bond, with an estimated bond dissociation energy of 533.5 kJ/mol. On the basis of bond length and electronegativity, the Schomaker-Stevenson rule suggests that the double bond form is very dominant (in contrast with POF3). The P=O bond does not utilize the d-orbitals on phosphorus as is commonly described in older textbooks as quantum chemical calculations have shown that d-orbitals are not involved in main group chemical bonding (see Hypervalent molecule). More modern texts favour a description which involves the donation of the lone pair electrons from oxygen p-orbitals to the antibonding phosphorus-chlorine bonds thus constituting π bonding.
With the freezing point of 1 °C and boiling point 106 °C, the liquid range is rather similar to water.
POCl3 reacts with water and alcohols to give hydrogen chloride and phosphoric acid or phosphate esters, respectively:
...
Wikipedia