Phosphites
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Systematic IUPAC name
Phosphite
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| 68617 | |||
| MeSH | Phosphorite | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| PO3− 3 |
|||
| Molar mass | 78.9720 g mol−1 | ||
| Related compounds | |||
|
Other anions
|
Phosphinite |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
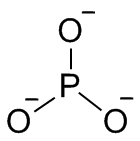
A phosphite in inorganic chemistry is a salt of phosphorous acid, H3PO3 and following the IUPAC naming recommendations the phosphite ion would be PO3−
3, a salt of P(OH)3.
Historically phosphite has referred to salts containing HPO2−
3 and this is because aqueous H3PO3 is not triprotic P(OH)3 but almost exclusively the diprotic HP(O)(OH)2 (IUPAC recommended name of phosphonic acid). The IUPAC-recommended name for the HPO2−
3 ion is phosphonate and this naming convention is becoming more common. In the US the IUPAC naming conventions for inorganic compounds are taught at high school, but not as a 'required' part of the curriculum. A well-known university-level textbook follows the IUPAC recommendations. In practise any reference to "phosphite" should be investigated carefully to determine which naming convention is being employed.
The term phosphite is also used to mean phosphite ester, an organophosphorus compound with the formula P(OR)3.
Salts containing PO3−
3 cannot be isolated from aqueous solutions of phosphorous acid. Only salts containing H
2PO−
3 or HPO2−
3 are produced. There are reports of a salt Na3PO3 in older literature and the use of sodium metal to remove the third hydrogen in H3PO3 is mentioned in a textbook. However, if PO3−
3 is produced in aqueous solution or dissolved it would form H
2PO−
3 or HPO2−
3 immediately. Na3PO3 is referred to in many textbooks on Internet sources, often as part of an exercise in naming inorganic compounds, but also as a misprint of the formula for the phosphate salt, Na3PO4.
...
Wikipedia