Peroxydisulfuric acid
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC names
μ-peroxido-bis(hydroxidodioxidosulfur)
peroxydisulfuric acid |
|
| Other names
Persulfuric acid, Peroxodisulfuric acid
|
|
| Identifiers | |
|
13445-49-3 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:29268 |
| ChemSpider |
22822 |
| PubChem | 24413 |
|
|
|
|
| Properties | |
| H2O8S2 | |
| Molar mass | 194.13 g·mol−1 |
| Appearance | Colourless solid |
| Melting point | 65 °C (149 °F; 338 K) (decomposes) |
| soluble | |
| Related compounds | |
|
Other cations
|
Potassium persulfate Sodium persulfate Ammonium persulfate |
|
Related compounds
|
Peroxymonosulfuric acid Pyrosulfuric acid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
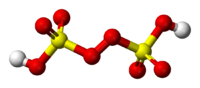
Peroxydisulfuric acid is the inorganic compound with the chemical formula H2S2O8. Also called Marshall's acid, it is sulfur oxoacid. In structural terms it can be written HO3SOOSO3H. It contains sulfur in its +6 oxidation state and a peroxide group. Its salts, commonly known as persulfates, are industrially important as powerful oxidizing agents.
The acid is prepared by the reaction of chlorosulfuric acid with hydrogen peroxide:
...
Wikipedia
