Pepto Bismol
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Pepto-Bismol |
| AHFS/Drugs.com | Multum Consumer Information |
| MedlinePlus | a607040 |
| Routes of administration |
Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.035.397 |
| Chemical and physical data | |
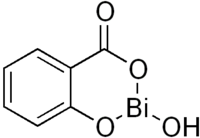
| Formula | C7H5BiO4 |
| Molar mass | 362.093 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Bismuth subsalicylate, sold under the brand name Pepto-Bismol, is an antacid medication used to treat temporary discomforts of the stomach and gastrointestinal tract, such as diarrhea, indigestion, heartburn and nausea. Commonly known as pink bismuth, it is also sometimes the active ingredient in Kaopectate.
Bismuth subsalicylate has the empirical chemical formula of C7H5BiO4, and it is a colloidal substance obtained by hydrolysis of bismuth salicylate (Bi{C6H4(OH)CO2}3). The actual structure is unknown and the formulation is only approximate.
As a derivative of salicylic acid, bismuth subsalicylate displays anti-inflammatory and bactericidal action. It also acts as an antacid.
There are some adverse effects. It can cause a black tongue and black stools in some users of the drug, when it combines with trace amounts of sulfur in saliva and the colon to form bismuth sulfide. Bismuth sulfide is a highly insoluble black salt, and the discoloration seen is temporary and harmless.
Long-term use (greater than 6 weeks) may lead to accumulation and toxicity. Some of the risks of salicylism can apply to the use of bismuth subsalicylate.
Children should not take medication with bismuth subsalicylate while recovering from influenza or chicken pox, as epidemiologic evidence points to an association between the use of salicylate-containing medications during certain viral infections and the onset of Reye's syndrome. For the same reason, it is typically recommended that nursing mothers not use medication containing bismuth subsalicylate because small amounts of the medication are excreted in breast milk and pose a theoretical risk of Reye's syndrome to nursing children.
...
Wikipedia
