Pentosan polysulfate
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| Pregnancy category |
|
| Routes of administration |
Oral, intramuscular, intra-articular, intraventricular |
| ATC code | |
| Pharmacokinetic data | |
| Excretion | Urine |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| ChEMBL | |
| Chemical and physical data | |
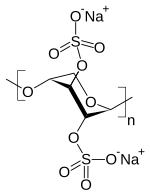
| Formula | (C5H6Na2O10S2)n |
Pentosan polysulfate (PPS, (1->4)-β-Xylan 2,3-bis(hydrogen sulfate) with a 4 O-methyl-α-D-glucuronate) is a semi-synthetic polysulfated xylan sold for the relief of various medical conditions including thrombi and interstitial cystitis in humans and osteoarthritis in dogs and horses.
The calcium salt of PPS was one of the first reported disease-modifying osteoarthritis drugs (DMOAD).
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a condition where patients struggle with symptoms of urinary frequency, urgency, pressure and/or pain, as well as nocturia (frequent urination at night), dyspareunia (painful intercourse), pain and/or discomfort while sitting in a car, while driving and/or traveling. PPS, as Elmiron, is the only oral medication approved by the U.S. Food and Drug Administration (FDA) for the treatment of IC. Elmiron is available as pills or as a direct infusion into the bladder. A review of four placebo-controlled studies concluded that PPS was significantly more effective than placebo for pain, urgency and frequency of urination, but not different from placebo for nocturia (needing to urinate during the night).
In IC, PPS is believed to work by providing a protective coating to the damaged bladder wall. PPS is similar in structure to the natural glycosaminoglycan coating of the inner lining of the bladder, and may replace or repair the lining, reducing its . This lining is important in preventing urinary toxins from irritating the underlying cell layers. Evidence for this mechanism was found by irritating the lining of the bladders of female rats with acrolein. If the rats were pre-treated with PPS, the damage was much less. Potassium sensitivity tests (PST) showed abnormal cell lining permeability in most patients with IC and indicated a significant reduction in permeability after successful PPS therapy (32-week trial of 300, 600, or 900 mg PPS/day). Another possible mechanism of PPS action in IC is by inhibiting the inflammatory response of the bladder cells, either by indirectly blocking the activity of mediators such as NF-κB, by preventing an influx of mast cells or by preventing mast cells releasing histamine.
...
Wikipedia
