Paraquat
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
1,1'-Dimethyl-4,4'-bipyridinium dichloride
|
|
| Other names
Paraquat dichloride; Methyl viologen dichloride; Crisquat; Dexuron; Esgram; Gramuron; Ortho Paraquat CL; Para-col; Pillarxone; Tota-col; Toxer Total; PP148; Cyclone; Gramixel; Gramoxone; Pathclear; AH 501.
|
|
| Identifiers | |
|
1910-42-5 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:34905 |
| ChEMBL |
ChEMBL458019 |
| ChemSpider |
15146 |
| ECHA InfoCard | 100.016.015 |
| 4552 | |
| PubChem | 15938 |
| UNII |
2KZ83GSS73 |
|
|
|
|
| Properties | |
| C12H14Cl2N2 | |
| Molar mass | 257.16 g·mol−1 |
| Appearance | Yellow solid |
| Odor | faint, ammonia-like |
| Density | 1.25 g/cm3 |
| Melting point | 175 to 180 °C (347 to 356 °F; 448 to 453 K) |
| Boiling point | > 300 °C (572 °F; 573 K) |
| High | |
| Vapor pressure | <0.0000001 mmHg (20°C) |
| Hazards | |
| Main hazards | Toxic, environmental hazard |
| Safety data sheet | Aldrich MSDS |
| GHS pictograms |
  
|
| H301, H311, H315, H319, H330, H335, H372, H410 | |
| P260, P273, P280, P284, P301+310, P305+351+338 | |
|
EU classification (DSD)
|
|
| R-phrases | R24/25, R26, R36/37/38, R48/25, R50/53 |
| S-phrases | (S1/2), S22, S28, S36/37/39, S45, S60, S61 |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
57 mg/kg (rat, oral) 120 mg/kg (mouse, oral) 25 mg/kg (dog, oral) 22 mg/kg (guinea pig, oral) |
|
LC50 (median concentration)
|
3 mg/m3 (mouse, 30 min respirable dust) 3 mg/m3 (guinea pig, 30 min respirable dust) |
|
LCLo (lowest published)
|
1 mg/m3 (rat, respirable dust, 6 hr) 6400 mg/m3 (rat, nonrespirable dust, 4 hr) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 0.5 mg/m3 (resp) [skin] |
|
REL (Recommended)
|
TWA 0.1 mg/m3 (resp) [skin] |
|
IDLH (Immediate danger)
|
1 mg/m3 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
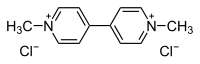
Paraquat (trivial name; /ˈpærəkwɑːt/) or N,N′-dimethyl-4,4′-bipyridinium dichloride (systematic name) is the organic compound with the chemical formula [(C6H7N)2]Cl2. It is classified as a viologen, a family of redox-active heterocycles of similar structure. Paraquat was manufactured by Chevron. This salt is one of the most widely used herbicides. It is quick-acting and non-selective, killing green plant tissue on contact. It is also toxic to human beings and animals. It is linked to development of Parkinson's disease. The name is derived from the para positions of the quaternary nitrogens. Quantities are sometimes expressed by cation mass alone (paraquat cation, paraquat ion); other salts (with other anions besides chloride) exist. In fact, its redox activity, which produces superoxide anions, is why it is toxic.
Pyridine is coupled by treatment with sodium in ammonia followed by oxidation. The resulting 4,4'-bipyridine is then methylated with chloromethane to give the title compound:
...
Wikipedia
