Nuclear pore complexes
| Nuclear pore | |
|---|---|
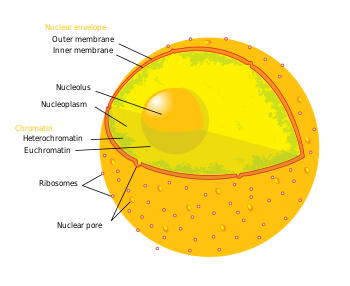
Diagram of human cell nucleus. Nuclear pore labeled at bottom left
|
|

Nuclear pore. Side view. 1. Nuclear envelope. 2. Outer ring. 3. Spokes. 4. Basket. 5. Filaments. (Drawing is based on electron microscopy images)
|
|
| Details | |
| Identifiers | |
| Latin | Porus nuclearis |
| TH | H1.00.01.2.01005 |
| FMA | 63148 |
|
Anatomical terminology
[]
|
|
Nuclear pores are large protein complexes that cross the nuclear envelope, which is the double membrane surrounding the eukaryotic cell nucleus. There are about an average of 2000 nuclear pore complexes (NPCs), in the nuclear envelope of a vertebrate cell, but it varies depending on cell type and the stage in the life cycle. The proteins that make up the nuclear pore complex are known as nucleoporins. About half of the nucleoporins typically contain solenoid protein domains—either an alpha solenoid or a beta-propeller fold, or in some cases both as separate structural domains. Each NPC contains at least 456 individual protein molecules and is composed of 30 distinct proteins (nucleoporins). The other half show structural characteristics typical of "natively unfolded" or intrinsically disordered proteins, i.e. they are highly flexible proteins that lack ordered secondary structure. These disordered proteins are the FG nucleoporins, so called because their amino-acid sequence contains many phenylalanine—glycine repeats.
Nuclear pore complexes allow the transport of molecules across the nuclear envelope. This transport includes RNA and ribosomal proteins moving from nucleus to the cytoplasm and proteins (such as DNA polymerase and lamins), carbohydrates, signaling molecules and lipids moving into the nucleus. It is notable that the nuclear pore complex (NPC) can actively conduct 1000 translocations per complex per second. Although smaller molecules simply diffuse through the pores, larger molecules may be recognized by specific signal sequences and then be diffused with the help of nucleoporins into or out of the nucleus. It has been recently shown that these nucleoporins have specific evolutionary conserved features encoded in their sequences that provide insight into how they regulate the transport of molecules through the nuclear pore. Nucleoporin-mediated transport is not directly energy requiring, but depends on concentrations gradients associated with the RAN cycle. Each of the eight protein subunits surrounding the actual pore (the outer ring) projects a spoke-shaped protein over the pore channel. The center of the pore often appears to contain a plug-like structure. It is yet unknown whether this corresponds to an actual plug or is merely cargo caught in transit.
...
Wikipedia
