Nitrogen peroxide
|
|
|||

Nitrogen dioxide at −196 °C, 0 °C, 23 °C, 35 °C, and 50 °C. (NO
2) converts to the colorless dinitrogen tetroxide (N 2O 4) at low temperatures, and reverts to NO 2 at higher temperatures. |
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Dinitrogen tetraoxide
|
|||
| Other names
Dinitrogen(II) oxide(-I)
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.031.012 | ||
| EC Number | 234-126-4 | ||
|
PubChem CID
|
|||
| RTECS number | QW9800000 | ||
| UNII | |||
| UN number | 1067 | ||
|
|||
|
|||
| Properties | |||
| N2O4 | |||
| Molar mass | 92.011 g/mol | ||
| Appearance | Colourless liquid/Orange gas | ||
| Density | 1.44246 g/cm3 (liquid, 21 °C) | ||
| Melting point | −11.2 °C (11.8 °F; 261.9 K) and decomposes to NO2 | ||
| Boiling point | 21.69 °C (71.04 °F; 294.84 K) | ||
| reacts | |||
| Vapor pressure | 96 kPa (20 °C) | ||
| −23.0·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.00112 | ||
| Structure | |||
| planar, D2h | |||
| zero | |||
| Thermochemistry | |||
|
Std molar
entropy (S |
304.29 J K−1 mol−1 | ||
|
Std enthalpy of
formation (ΔfH |
+9.16 kJ/mol | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
|
EU classification (DSD) (outdated)
|
|
||
| R-phrases (outdated) | R26, R34 | ||
| S-phrases (outdated) | (S1/2), S9, S26, S28, S36/37/39, S45 | ||
| NFPA 704 | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
|
Nitrous oxide Nitric oxide Dinitrogen trioxide Nitrogen dioxide Dinitrogen pentoxide |
|||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide.
Dinitrogen tetroxide is a powerful oxidizer that is hypergolic (spontaneously reacts) upon contact with various forms of hydrazine, which has made the pair a common bipropellant for rockets.
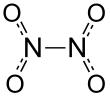
Dinitrogen tetroxide could be regarded as two nitro groups (-NO2) bonded together. It forms an equilibrium mixture with nitrogen dioxide. The molecule is planar with an N-N bond distance of 1.78 Å and N-O distances of 1.19 Å. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1.45 Å.
Unlike NO2, N2O4 is diamagnetic since it has no unpaired electrons. The liquid is also colorless but can appear as a brownish yellow liquid due to the presence of NO2 according to the following equilibrium:
Higher temperatures push the equilibrium towards nitrogen dioxide. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide.
Nitrogen tetroxide is made by the catalytic oxidation of ammonia: steam is used as a diluent to reduce the combustion temperature. In the first step, the ammonia is oxidized into nitric oxide:
Most of the water is condensed out, and the gases are further cooled; the nitric oxide that was produced is oxidized to nitrogen dioxide, which is then dimerized into nitrogen tetroxide:
...
Wikipedia