Nilotinib
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Tasigna |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608002 |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 30% |
| Protein binding | 98% |
| Metabolism | Hepatic (mostly CYP3A4-mediated) |
| Biological half-life | 15-17 hours |
| Excretion | Faeces (93%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.166.395 |
| Chemical and physical data | |
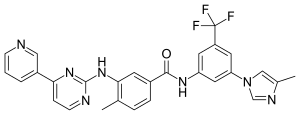
| Formula | C28H22F3N7O |
| Molar mass | 529.5245 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Nilotinib (AMN107, trade name Tasigna), in the form of the hydrochloride monohydrate salt, is a small-molecule tyrosine kinase inhibitor approved for the treatment of imatinib-resistant chronic myelogenous leukemia. Structurally related to imatinib, it was developed based on the structure of the Abl-imatinib complex to address imatinib intolerance and resistance. Nilotinib is a selective Bcr-Abl kinase inhibitor that is 10-30 fold more potent than imatinib in inhibiting Bcr-Abl tyrosine kinase activity and proliferation of Bcr-Abl expressing cells. Nolitinib was developed by Novartis and is sold under the trade name Tasigna.
It is FDA- (29 October 2007),EMA- (29 September 2009),MHRA- (19 November 2007) and TGA- (17 January 2008) approved for use as a treatment for Philadelphia chromosome (Ph+)-positive chronic myelogenous leukaemia.
The drug carries a black box warning for possible heart complications.
In June 2006, a phase I clinical trial found nilotinib has a relatively favorable safety profile and shows activity in cases of CML resistant to treatment with imatinib, another tyrosine kinase inhibitor currently used as a first-line treatment. In that study 92% of patients (already resistant or unresponsive to imatinib) achieved normal white blood cell counts after five months of treatment.
Novartis announced on April 11, 2011 that it is discontinuing a phase III trial of Tasigna (nilotinib) for investigational use in the first-line treatment of gastrointestinal stromal tumor (GIST) based on the recommendation of an independent data monitoring committee. Interim results showed Tasigna is unlikely to demonstrate superiority compared to Novartis's Gleevec (imatinib)*, the current standard of care in this setting.
...
Wikipedia
