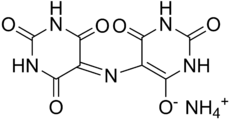
Murexide
 |
|
| Names | |
|---|---|
|
IUPAC name
Ammonium 2,6-dioxo-5-[(2,4,6-trioxo-5-hexahydropyrimidinylidene)amino]-3H-pyrimidin-4-olate
|
|
| Other names
Purpuric acid ammonium salt
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.019.334 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C8H8N6O6 | |
| Molar mass | 284.19 g·mol−1 |
| Hazards | |
| R-phrases | R20 R21 R22 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Murexide (NH4C8H4N5O6, or C8H5N5O6·NH3), also called ammonium purpurate or MX, is the ammonium salt of purpuric acid. It may be prepared by heating alloxantin in ammonia gas to 100 °C, or by boiling uramil (5-aminobarbituric acid) with mercury oxide. W.N. Hartley found considerable difficulty in obtaining specimens of murexide sufficiently pure to give concordant results when examined by means of their absorption spectra, and consequently devised a new method of preparation for murexide. In this process alloxantin is dissolved in a large excess of boiling absolute alcohol, and dry ammonia gas is passed into the solution for about three hours. The solution is then filtered from the precipitated murexide, which is washed with absolute alcohol and dried. The salt obtained in this way is in the anhydrous state. It may also be prepared by digesting alloxan with alcoholic ammonia at about 78 °C; the purple solid so formed is easily soluble in water, and the solution produced is indistinguishable from one of murexide.
Murexide in its dry state has the appearance of a reddish purple powder, slightly soluble in water. In solution, its color ranges from yellow in strong acidic pH through reddish-purple in weakly acidic solutions to blue-purple in alkaline solutions. The pH for titration of calcium is 11.3.
Justus von Liebig and Friedrich Wöhler in Giessen, Germany, had investigated the purple product, murexide, obtained from snake excrement in the 1830s but this was not an abundant raw material and a method of using it as a dyestuff was not established at that time. In the 1850s, French colorists and dye-producers, such as Depoully in Paris, succeeded in making murexide from abundant South American guano, and of applying it to natural fibers. It was then widely adopted in Britain, France and Germany.
...
Wikipedia
