Minocycline
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Minocin, Minomycin, Akamin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682101 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Metabolism | Liver |
| Biological half-life | 11–22 hours |
| Excretion | mostly fecal, rest renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.226.626 |
| Chemical and physical data | |
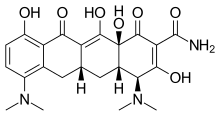
| Formula | C23H27N3O7 |
| Molar mass | 457.477 |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Minocycline is a broad-spectrum tetracycline antibiotic, and has a broader spectrum than the other members of the group. It is a bacteriostatic antibiotic, classified as a long-acting type. As a result of its long half-life it generally has serum levels 2–4 times that of the simple water-soluble tetracyclines.
Minocycline is the most lipid-soluble of the tetracycline-class antibiotics, giving it the greatest penetration into the prostate and brain, but also the greatest amount of central nervous system (CNS)-related side effects, such as vertigo. A common side effect is diarrhea. Uncommon side effects (with prolonged therapy) include skin discolouration and autoimmune disorders that are not seen with other drugs in the class.
Minocycline is a relatively poor tetracycline-class antibiotic choice for urinary pathogens sensitive to this antibiotic class, as its solubility in water and levels in the urine are less than all other tetracyclines. Minocycline is metabolized by the liver and has poor urinary excretion.
Minocycline was patented in 1961 and came into commercial use in 1971. is not a naturally occurring antibiotic, but was synthesized semi-synthetically from natural tetracycline antibiotics by Lederle Laboratories in 1966, and marketed by them under the brand name Minocin.
Minocycline and doxycycline are frequently used for the treatment of acne vulgaris. Both of these closely related antibiotics have similar levels of efficacy, although doxycycline has a slightly lower risk of adverse side effects. Historically, minocycline has been a very effective treatment for acne vulgaris. However, acne that is caused by antibiotic resistant bacteria is a growing problem in many countries. In Europe and North America, a significant number of acne patients no longer respond well to treatment with tetracycline family antibiotics (e.g. tetracycline, doxycycline and minocycline) because their acne symptoms are caused by bacteria (primarily Propionibacterium acnes) that are resistant to these antibiotics.
Minocycline is also used for other skin infections such as MRSA as well as Lyme disease, as the one pill twice daily 100 mg dosage is far easier for patients than the four times a day required with tetracycline or oxytetracycline. Its activity against Lyme disease is enhanced by its superior ability to cross the blood-brain barrier.
...
Wikipedia
