Methylone
 |
|
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Common: oral, insufflation Uncommon: IV or IM injection, rectal |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
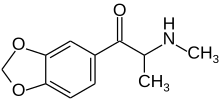
| Formula | C11H13NO3 |
| Molar mass | 207.23 g/mol |
| 3D model (Jmol) | |
| Solubility in water | 357 mg/mL (20 °C) |
|
|
|
|
|
|
|
Methylone (also known as "3,4-methylenedioxy-N-methylcathinone", "MDMC", "βk-MDMA" and by the slang term "M1") is an empathogen and stimulant psychoactive drug. It is a member of the substituted amphetamine, substituted cathinone and substituted methylenedioxyphenethylamine classes.
Methylone is the substituted cathinone analog of MDMA and the 3,4-methylenedioxy analog of methcathinone. The only structural difference of methylone with respect to MDMA is the substitution of 2 hydrogen atoms by 1 oxygen atom in the β position of the phenethylamine core, forming a ketone group.
Methylone was first synthesized by the chemists Peyton Jacob III and Alexander Shulgin in 1996 for potential use as an antidepressant. Starting around 2004, methylone has been sold for recreational use, taking advantage of the absence of legal prohibition of this compound in many countries.
Methylone substitutes for MDMA in rats trained to discriminate MDMA from saline. Methylone does not substitute for amphetamine or for the hallucinogenic DOM in animals trained to discriminate between these drugs and saline. Further, also in common with MDMA, methylone acts on monoaminergic systems. In vitro, methylone has one third the potency of MDMA at inhibiting platelet serotonin accumulation and about the same in its inhibiting effects on the dopamine and noradrenaline transporters.
In spite of these behavioral and pharmacological similarities between methylone and MDMA, the observed subjective effects of both drugs are not completely identical. Alexander Shulgin wrote of the former:
...
Wikipedia
