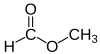
Methyl formate
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Methyl formate
|
|||
|
Systematic IUPAC name
Methyl methanoate
|
|||
| Other names
R-611
|
|||
| Identifiers | |||
|
107-31-3 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:77699 |
||
| ChEMBL |
ChEMBL295026 |
||
| ChemSpider |
7577 |
||
| ECHA InfoCard | 100.003.166 | ||
| PubChem | 7865 | ||
| UNII |
1MPH591FTG |
||
|
|||
|
|||
| Properties | |||
| C2H4O2 | |||
| Molar mass | 60.05 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | pleasant | ||
| Density | 0.98 g/cm3 | ||
| Melting point | −100 °C (−148 °F; 173 K) | ||
| Boiling point | 32 °C (90 °F; 305 K) | ||
| 30% (20°C) | |||
| Vapor pressure | 476 mmHg (20°C) | ||
| -32.0·10−6 cm3/mol | |||
| Hazards | |||
| Safety data sheet | Oxford MSDS | ||
|
EU classification (DSD)
|
Highly flammable (F+); Harmful (Xn) | ||
| Flash point | −19 °C; −2 °F; 254 K | ||
| Explosive limits | 4.5%-23% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
1622 mg/kg (oral, rabbit) | ||
|
LCLo (lowest published)
|
50,000 ppm (guinea pig, 20 min) | ||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 100 ppm (250 mg/m3) | ||
|
REL (Recommended)
|
TWA 100 ppm (250 mg/m3) ST 150 ppm (375 mg/m3) | ||
|
IDLH (Immediate danger)
|
4500 ppm | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of an ester, it is a colorless liquid with an ethereal odour, high vapor pressure, and low surface tension. It is a precursor to many other compounds of commercial interest.
In some cases people have found that the pleasant smell is of lemons or lemonade.
In the laboratory, methyl formate can be produced by the condensation reaction of methanol and formic acid, as follows:
Industrial methyl formate, however, is usually produced by the combination of methanol and carbon monoxide (carbonylation) in the presence of a strong base, such as sodium methoxide:
This process, practiced commercially by BASF among other companies gives 96% selectivity toward methyl formate. The catalyst for this process is sensitive to water, which can be present in the carbon monoxide feedstock, which is commonly derived from synthesis gas. Very dry carbon monoxide is, therefore, essential.
Methyl formate is used primarily to manufacture formamide, dimethylformamide, and formic acid. These compounds are precursors or building blocks for many useful derivatives.
Because of its high vapor pressure, it is used for quick-drying finishes and as a blowing agent for some polyurethane foam applications and as a replacement for CFCs, HCFCs, and HFCs. Methyl formate has zero ozone depletion potential and zero global warming potential. It is also used as an insecticide.
...
Wikipedia