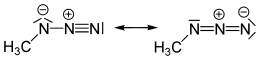
Methyl azide
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Azidomethane
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChemSpider | |||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| CH3N3 | |||
| Molar mass | 57.05 | ||
| Appearance | white powder | ||
| slightly soluble | |||
| Solubility | alkane, ether | ||
| Explosive data | |||
| Shock sensitivity | High | ||
| Friction sensitivity | High | ||
| Hazards | |||
| Main hazards | Highly explosive | ||
| Related compounds | |||
|
Related compounds
|
Hydrazoic acid, Chlorine azide, Ethyl azide | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Methyl azide is a covalent molecule related to hydrazoic acid and other alkyl azides.
It can be prepared by a methylation of sodium azide. The first synthesis was reported in 1905. It decomposes in a first-order reaction:
Methyl azide might be a potential precursor in the synthesis of prebiotic molecules via nonequilibrium reactions on interstellar ices initiated by energetic galactic cosmic rays (GCR) and photons.
Methyl azide is stable at ambient temperature but may explode when heated. Presence of mercury increases the sensitivity to shock and spark. Incompatible with (dimethyl malonate + sodium methylate); mercury; methanol; sodium azide; dimethyl sulfate; sodium hydroxide; hydrogen azide. When heated to decomposition it emits toxic fumes of NOx.
...
Wikipedia


