Metallothionein
| Metallothionein superfamily (plant) |

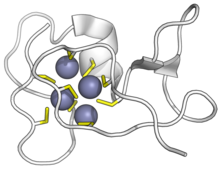
Beta-E-domain of wheat Ec-1 metallothionein bound to zinc ions. Cysteines in yellow, zinc in purple. ( PDB: 2KAK) |
| Identifiers |
| Symbol |
Metallothionein_sfam |
| Pfam |
PF00131 |
| InterPro |
IPR003019 |
|
|
Metallothionein (MT) is a family of cysteine-rich, low molecular weight (MW ranging from 500 to 14000 Da) proteins. They are localized to the membrane of the Golgi apparatus. MTs have the capacity to bind both physiological (such as zinc, copper, selenium) and xenobiotic (such as cadmium, mercury, silver, arsenic) heavy metals through the thiol group of its cysteine residues, which represent nearly 30% of its constituent amino acid residues.
MT was discovered in 1957 by Vallee and Margoshe from purification of a Cd-binding protein from horse (equine) renal cortex. MTs function is not clear, but experimental data suggest MTs may provide protection against metal toxicity, be involved in zinc and copper regulation, and provide protection against oxidative stress. There are four main isoforms expressed in humans (family 1, see chart below): MT1 (subtypes A, B, E, F, G, H, L, M, X), MT2, MT3, and MT4. In the human body, large quantities are synthesised primarily in the liver and kidneys. Their production is dependent on availability of the dietary minerals such as zinc, copper, and selenium, as well as the amino acids histidine and cysteine.
...
Wikipedia