Mercury(II) thiocyanate
 |
|
 |
|
| Names | |
|---|---|
| Other names
Mercuric thiocyanate
Mercuric sulfocyanate |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ECHA InfoCard | 100.008.886 |
| EC Number | 209-773-0 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
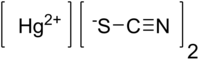
| Hg(SCN)2 | |
| Molar mass | 316.755 g/mol |
| Appearance | White monoclinic powder |
| Odor | odorless |
| Density | 3.71 g/cm3, solid |
| Melting point | 165 °C (329 °F; 438 K) (decomposes) |
| 0.069 g/100 mL | |
| Solubility | Soluble in dilute hydrochloric acid, KCN, ammonia slightly soluble in alcohol, ether |
| −96.5·10−6 cm3/mol | |
| Hazards | |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
46 mg/kg (rat, oral) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Mercury(II) thiocyanate (Hg(SCN)2) is an inorganic chemical compound, the coordination complex of Hg2+ and the thiocyanate anion. It is a white powder. It will produce a large, winding “snake” when ignited, an effect known as the Pharaoh's serpent.
The first synthesis of mercury thiocyanate was probably completed in 1821 by Jöns Jacob Berzelius:
Evidence for the first pure sample was presented in 1866 prepared by a chemist named Otto Hermes. It is prepared by treating solutions containing mercury(II) and thiocyanate ions. The low solubility product of mercury thiocyanate causes it to precipitate from solution. Most syntheses are achieved by precipitation:
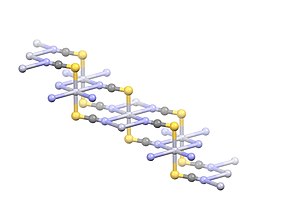
The compound adopts a polymeric structure with Hg2+ centers linearly coordinated to two S atoms with a distance of 2.381 Å. Four weak Hg2+--N interactions are indicated with distances of 2.81 Å.
Mercury thiocyanate was formerly used in pyrotechnics causing an effect known as the Pharaoh's serpent or Pharaoh's snake. When the compound is in the presence of a strong enough heat source, a rapid exothermic reaction is started which produces a large mass of coiling serpent-like solid. An inconspicuous flame which is often blue but can also occur in yellow/orange accompanies the combustion. The resulting solid can range from dark graphite grey to light tan in color with the inside generally much darker than the outside.
The reaction has several stages as follows:
Igniting mercury thiocyanate causes it to form an insoluble brown mass that is primarily carbon nitride, C3N4. Mercury sulphide and carbon disulphide are also produced.
2Hg(SCN)2 → 2HgS + CS2 + C3N4
Carbon disulphide combusts to carbon dioxide and sulphur dioxide:
CS2 + 3O2 → CO2 + 2SO2
The heated C3N4 partially breaks down to form nitrogen gas and cyanogen:
2C3N4 → 3(CN)2 + N2
Mercury sulphide reacts with oxygen to form mercury vapour and sulphur dioxide. If the reaction is performed inside a container, a grey film of mercury coating on its inner surface can be observed.
...
Wikipedia

