Merbromin
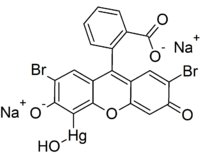 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
dibromohydroxymercurifluorescein
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.486 |
| EC Number | 204-933-6 |
| KEGG | |
| UNII | |
|
|
|
|
| Properties | |
| C20H8Br2HgNa2O6 | |
| Molar mass | 750.66 g·mol−1 |
| Appearance | dark green solid |
| Pharmacology | |
| D08AK04 (WHO) | |
| Hazards | |
| Main hazards | Toxic, dangerous for the environment |
| R-phrases (outdated) | R26 R27 R28 R33 R50 R53 |
| S-phrases (outdated) | S13 S28 S36 S45 S60 S61 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Merbromin (marketed as Mercurochrome, Merbromine, Sodium mercurescein, Asceptichrome, Supercrome, Brocasept and Cinfacromin) is a topical antiseptic used for minor cuts and scrapes. Merbromin is an organomercuric disodium salt compound and a fluorescein. It is readily available in most countries but, because of its mercury content, it is no longer sold in Switzerland, France, Germany, and the United States.
Merbromin's best-known use is as a topical antiseptic to treat minor wounds, burns, and scratches. It is also used in the antisepsis of the umbilical cord and the antisepsis of wound of difficult scar formation, like neuropathic ulcers, and diabetic foot sores. When applied on a wound, it stains the skin a distinctive carmine red, which can last up to 2 weeks through repeated washings. It is useful on infections of the finger or toe nails because of its permanence, and lethality to bacteria. It was known in many households as "monkey blood."
The U.S. Food and Drug Administration in 1998 classified merbromin as "not Generally Recognized as Safe" together with a multitude of other active compounds, based on the absence of interest on the part of pharmaceutical companies in funding new studies or updated supporting information, due to the high costs of said studies in comparison to sales, rather than due to being toxic.
In the United States, its use has been superseded by other agents (e.g., povidone iodine, benzalkonium chloride, chloroxylenol). It is still an important antiseptic, particularly in developing nations, due to its “unbelievably low cost.”
Merbromin is also used as a biological dye to mark tissue margins and as a metal dye in industrial dye penetrant inspection to detect metal fractures.
Merbromin is synthesized by combining dibromofluorescein with mercuric acetate and sodium hydroxide or alternatively, through action of the mercuric acetate upon or (combining with) sodium dibromofluorescein. Because of its anionic character, it is chemically incompatible with acids, the majority of alkaloid salts and most local anesthetics.
...
Wikipedia
