Memantine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Originally, Axura, Ebixa, Namenda; many generic names worldwide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604006 |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | N06DX01 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Metabolism | Hepatic (<10%) |
| Biological half-life | 60–100 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number |
19982-08-2 |
| PubChem (CID) | 4054 |
| IUPHAR/BPS | 4253 |
| DrugBank |
DB01043 |
| ChemSpider |
3914 |
| UNII |
W8O17SJF3T |
| KEGG |
D08174 |
| ChEMBL |
CHEMBL807 |
| Chemical and physical data | |
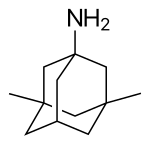
| Formula | C12H21N |
| Molar mass | 179.3 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Memantine is the first in a novel class of Alzheimer's disease medications acting on the glutamatergic system by blocking NMDA receptors. It was first synthesized by Eli Lilly and Company in 1968 as a potential agent to treat diabetes; the NMDA activity was discovered in the 1980s.
Memantine is marketed under the brands Namenda / Auxura / Ebixa and Memary among others. Memantine has been shown to have a modest effect in moderate-to-severe Alzheimer's disease and in dementia with Lewy bodies. Despite years of research, there is little evidence of effect on mild Alzheimer's disease.
Memantine is approved by the U.S. FDA and the European Medicines Agency for treatment of moderate-to-severe Alzheimer's disease, and has now received a limited recommendation by the UK's National Institute for Health and Care Excellence for patients who fail other treatment options. Within the new guidance memantine is recommended as an option for managing Alzheimer’s disease for people with: moderate Alzheimer’s disease who are intolerant of or have a contraindication to AChE (acetylcholinesterase) inhibitors or those with severe Alzheimer’s disease.
Memantine has been associated with a moderate decrease in clinical deterioration with only a small positive effect on cognition, mood, behavior, and the ability to perform daily activities in moderate to severe Alzheimer's disease. There does not appear to be any benefit in mild disease.
It has shown promising results in studies for treatment of obsessive compulsive disorder,generalized anxiety disorder, as an augmentation therapy for anxiety disorders,ADHD, as well as to help slowing down or even reversing the tolerance development to opioids.
Memantine is, in general, well-tolerated. Common adverse drug reactions (≥1% of patients) include confusion, dizziness, drowsiness, headache, insomnia, agitation, and/or hallucinations. Less common adverse effects include vomiting, anxiety, hypertonia, cystitis, and increased libido. It has been reported to induce reversible neurological impairment in multiple sclerosis patients, which led to the halt of an ongoing clinical trial. Though exceedingly rare, extrapyramidal side effects (such as dystonic reactions, etc.) may occur, in particular, in the younger population.
...
Wikipedia
