Mebeverine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Colofac, Duspamen, Duspatalin and others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.018.546 |
| Chemical and physical data | |
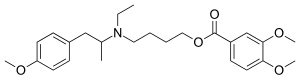
| Formula | C25H35NO5 |
| Molar mass | 429.6 g/mol |
| 3D model (Jmol) | |
| Chirality | Racemic mixture |
|
|
|
|
Mebeverine is a drug whose major therapeutic role is in the treatment of irritable bowel syndrome (IBS) and the associated abdominal cramping. It works by relaxing the muscles in and around the gut. It is a musculotropic antispasmodic drug without anticholinergic side effects. The drug is also indicated for treatment of gastrointestinal spasm secondary to organic disorder.
Spastic functional disturbances of the colon:
Mebeverine should be taken 20 minutes before meals.
Mebeverine is an antimuscarinic. It is also an inhibitor of calcium-depot replenishment. Musculotropic compounds act directly on the gut muscles at the cellular level to relax them. This relieves painful muscle spasms of the gut, without affecting its normal motility. Mebeverine is used to relieve symptoms of irritable bowel syndrome and related intestinal disorders that are the result of spasms in the intestinal muscles. These include colicky abdominal pain and cramps, diarrhoea alternating with constipation and flatulence (wind).
Side effects may include:
Since 1978, 21 cases of severe adverse reactions to mebeverine were reported in the Netherlands. Most reactions consisted of urticaria or maculopapular rash, sometimes accompanied by fever, polyarthritis, thrombocytopenia or angioedema.
Very rarely, people taking this medicine may develop allergic reactions.
Mebeverine passes into breast milk, but the amount is considered too small to be harmful to a nursing infant.
Mebeverine is unlikely to affect the ability to operate machinery or to drive, yet not completely out of the question.
It was first registered in 1965.
Mebeverine is a generic drug and is available internationally under many brand names.
...
Wikipedia
