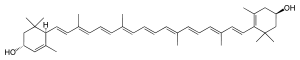
Lutein
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
β,ε-carotene-3,3'-diol
|
|
| Other names
Luteine; trans-lutein; 4-[18-(4-Hydroxy-2,6,6-trimethyl-1-cyclohexenyl)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-3,5,5-trimethyl-cyclohex-2-en-1-ol
|
|
| Identifiers | |
|
127-40-2 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:28838 |
| ChEMBL |
ChEMBL173929 |
| ChemSpider |
4444655 |
| ECHA InfoCard | 100.004.401 |
| E number | E161b (colours) |
| PubChem | 5281243 |
| UNII |
X72A60C9MT |
|
|
|
|
| Properties | |
| C40H56O2 | |
| Molar mass | 568.871 g/mol |
| Appearance | Red-orange crystalline solid |
| Melting point | 190 °C (374 °F; 463 K) |
| Insoluble | |
| Solubility in fats | Soluble |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Lutein (/ˈluːtiː.ᵻn/ or /ˈluːtiːn/; from Latin luteus meaning "yellow") is a xanthophyll and one of 600 known naturally occurring carotenoids. Lutein is synthesized only by plants and like other xanthophylls is found in high quantities in green leafy vegetables such as spinach, kale and yellow carrots. In green plants, xanthophylls act to modulate light energy and serve as agents to deal with triplet chlorophyll (an excited form of chlorophyll), which is overproduced at very high light levels, during photosynthesis. See xanthophyll cycle for this topic.
Lutein is obtained by animals directly or indirectly, from plants. Lutein is apparently employed by animals as an antioxidant and for blue light absorption. Lutein is found in egg yolks and animal fats. In addition to coloring yolks, lutein causes the yellow color of chicken skin and fat, and is used in chicken feed for this purpose. The human retina accumulates lutein and zeaxanthin. The latter predominates at the macula lutea while lutein predominates elsewhere in the retina. There, it may serve as a photoprotectant for the retina from the damaging effects of free radicals produced by blue light. Lutein is isomeric with zeaxanthin, differing only in the placement of one double bond.
...
Wikipedia
