Lexapro
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | |
| Trade names | Cipralex, Lexapro and many others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603005 |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% |
| Protein binding | ~56% |
| Metabolism | Liver, specifically the enzymes CYP3A4 and CYP2C19 |
| Biological half-life | 27–32 hours |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
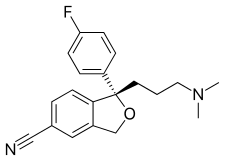
| Formula | C20H21FN2O |
| Molar mass | 324.392 g/mol (414.43 as oxalate) |
| 3D model (JSmol) | |
|
|
|
|
|
|
|
Escitalopram, also known by the brand names Lexapro and Cipralex among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is approved by the U.S. Food and Drug Administration (FDA) for the treatment of adults and children over 12 years of age with major depressive disorder (MDD) or generalized anxiety disorder (GAD). Escitalopram is the (S)-stereoisomer (Left-enantiomer) of the earlier Lundbeck drug citalopram, hence the name escitalopram. Whether escitalopram exhibits superior therapeutic properties to citalopram or merely represents an example of "evergreening" is controversial.
Escitalopram has FDA approval for the treatment of major depressive disorder in adolescents and adults, and generalized anxiety disorder in adults. In European countries and Australia, it is approved for depression (MDD) and certain anxiety disorders: general anxiety disorder (GAD), social anxiety disorder (SAD), obsessive-compulsive disorder (OCD), and panic disorder with or without agoraphobia.
Escitalopram was approved by regulatory authorities for the treatment of major depressive disorder on the basis of four placebo controlled, double-blind trials, three of which demonstrated a statistical superiority over placebo.
...
Wikipedia
