Laughing gas
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Dinitrogen monoxide
|
|
| Other names
Laughing gas, Sweet air, Protoxide of nitrogen, Hyponitrous oxide
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| 8137358 | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.030.017 |
| E number | E942 (glazing agents, ...) |
| 2153410 | |
| KEGG | |
|
PubChem CID
|
|
| RTECS number | QX1350000 |
| UNII | |
| UN number | 1070 (compressed) 2201 (liquid) |
|
|
|
|
| Properties | |
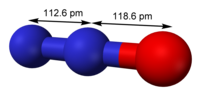
| N 2O |
|
| Molar mass | 44.013 g/mol |
| Appearance | colorless gas |
| Density | 1.977 g/L (gas) |
| Melting point | −90.86 °C (−131.55 °F; 182.29 K) |
| Boiling point | −88.48 °C (−127.26 °F; 184.67 K) |
| 1.5 g/L (15 °C) | |
| Solubility | soluble in alcohol, ether, sulfuric acid |
| log P | 0.35 |
| Vapor pressure | 5150 kPa (20 °C) |
| −18.9·10−6 cm3/mol | |
|
Refractive index (nD)
|
1.000516 (0 °C, 101,325 kPa) |
| Structure | |
| linear, C∞v | |
| 0.166 D | |
| Thermochemistry | |
|
Std molar
entropy (S |
219.96 J K−1 mol−1 |
|
Std enthalpy of
formation (ΔfH |
+82.05 kJ mol−1 |
| Pharmacology | |
| N01AX13 (WHO) | |
|
|
| Inhalation | |
| Pharmacokinetics: | |
| 0.004% | |
| 5 minutes | |
| Respiratory | |
| Hazards | |
| Safety data sheet | Ilo.org, ICSC 0067 |
| NFPA 704 | |
| Flash point | Nonflammable |
| Related compounds | |
|
Nitric oxide Dinitrogen trioxide Nitrogen dioxide Dinitrogen tetroxide Dinitrogen pentoxide |
|
|
Related compounds
|
Ammonium nitrate Azide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Nitrous oxide, commonly known as laughing gas or nitrous, is a chemical compound, an oxide of nitrogen with the formula N
2O. At room temperature, it is a colorless, odorless non-flammable gas, with a slightly sweet taste. At elevated temperatures, nitrous oxide is a powerful oxidizer similar to molecular oxygen.
Nitrous oxide has significant medical uses, especially in surgery and dentistry, for its anaesthetic and analgesic effects. Its name "laughing gas" is due to the euphoric effects of inhaling it, a property that has led to its recreational use as a dissociative anaesthetic. It is also used as an oxidizer in rocket propellants, and in motor racing to increase the power output of engines.
Nitrous oxide occurs in small amounts in the atmosphere, but has been found recently to be a major scavenger of stratospheric ozone, with impact comparable to that of CFCs. It is estimated that 30% of the N
2O in the atmosphere is the result of human activity, chiefly agriculture.
Nitrous oxide can be used as an oxidizer in a rocket motor. This has the advantages over other oxidisers in that it is not only non-toxic, but also, due to its stability at room temperature, easy to store and relatively safe to carry on a flight. As a secondary benefit it can be readily decomposed to form breathing air. Its high density and low storage pressure (when maintained at low temperature) enable it to be highly competitive with stored high-pressure gas systems.
...
Wikipedia

