Largactil
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Largactil, Thorazine, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682040 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral (tablets and syrup available), rectal, IM, IV infusion |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 10–80% (Oral; large interindividual variation) |
| Protein binding | 90–99% |
| Metabolism | Liver, mostly CYP2D6-mediated |
| Biological half-life | 30 hours |
| Excretion | Urine (43–65% in 24 hrs) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.000.042 |
| Chemical and physical data | |
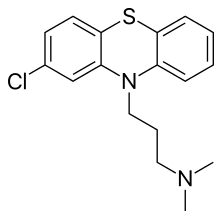
| Formula | C17H19ClN2S |
| Molar mass | 318.86 g/mol (free base) 355.33 g/mol (hydrochloride) |
| 3D model (Jmol) | |
|
|
|
|
Chlorpromazine (CPZ), marketed under the trade names Thorazine and Largactil among others, is an antipsychotic medication. It is primarily used to treat psychotic disorders such as schizophrenia. Other uses include the treatment of bipolar disorder, attention deficit hyperactivity disorder, nausea and vomiting, anxiety before surgery, and hiccups that do not improve following other measures. It can be given by mouth, by injection into a muscle, or into a vein.
Common side effects include movement problems, sleepiness, dry mouth, low blood pressure upon standing, and increased weight. Serious side effects may include the potentially permanent movement disorder tardive dyskinesia, neuroleptic malignant syndrome, and low white blood cell levels. In older people with psychosis as a result of dementia it may increase the risk of dying. It is unclear if it is safe for use in pregnancy. Chlorpromazine is in the typical antipsychotic class. Its mechanism of action is not entirely clear but believed to be related to its ability as a dopamine antagonist. It also has anti-serotonergic and antihistaminergic properties.
...
Wikipedia
