L-phenylalanine

L-Phenylalanine
|
|
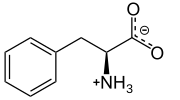
L-Phenylalanine at physiological pH
|
|
 3D phenylalanine model
|
|
| Names | |
|---|---|
|
IUPAC name
(S)-2-Amino-3-phenylpropanoic acid
|
|
| Identifiers | |
|
150-30-1 (DL) 63-91-2 (L) |
|
| ChEBI |
CHEBI:58095 |
| ChEMBL |
ChEMBL301523 |
| ChemSpider |
5910 |
| DrugBank |
DB00120 |
| ECHA InfoCard | 100.000.517 |
| 3313 | |
| KEGG |
D00021 |
| PubChem | 994 |
| UNII |
8P946UF12S |
|
|
| Properties | |
| C9H11NO2 | |
| Molar mass | 165.19 g/mol |
| Acidity (pKa) | 1.83 (carboxyl), 9.13 (amino) |
| Hazards | |
| Safety data sheet | See: data page |
| NFPA 704 | |
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Phenylalanine (US: /ˌfɛnəlˈæləniːn/, UK: /ˌfiːnaɪlˈæləniːn/; abbreviated as Phe or F) is an α-amino acid with the formula C
9H
11NO
2. It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The L-isomer is used to biochemically form proteins, coded for by DNA. The codons for L-phenylalanine are UUU and UUC. Phenylalanine is a precursor for tyrosine; the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline); and the skin pigment melanin.
...
Wikipedia

