Jakafi
 |
|
| Clinical data | |
|---|---|
| Trade names | Jakafi, Jakavi |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612006 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 95% |
| Protein binding | 97% |
| Metabolism | Hepatic (mainly CYP3A4-mediated) |
| Biological half-life | 2.8-3 hours |
| Excretion | Urine (74%), faeces (22%) |
| Identifiers | |
|
|
| Synonyms | INCB018424, INC424 |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
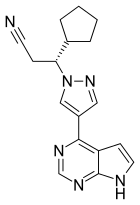
| Formula | C17H18N6 |
| Molar mass | 306.37 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Ruxolitinib (trade names Jakafi JAK-ə-fye and Jakavi) is a drug for the treatment of intermediate or high-risk myelofibrosis, a type of myeloproliferative disorder that affects the bone marrow, and for polycythemia vera (PCV) when there has been an inadequate response to or intolerance of hydroxyurea.
Ruxolitinib is a janus kinase inhibitor with selectivity for subtypes JAK1 and JAK2 of this enzyme. Ruxolitinib inhibits dysregulated JAK signaling associated with myelofibrosis. JAK1 and JAK2 recruit signal transducers and activators of transcription (STATs) to cytokine receptors leading to modulation of gene expression.
Side effects include thrombocytopenia (low blood platelet count), anemia (low red blood cell count) and neutropenia; risk of infection; symptom exacerbation if the medication is interrupted or discontinued; and non-melanoma skin cancer.
Immunologic side effects have included herpes zoster (shingles) and case reports of opportunistic infections. Metabolic side effects have included weight gain. Laboratory abnormalities have included alanine transaminase (ALT) abnormalities, aspartate transaminase (AST) abnormalities, and mildly elevated cholesterol levels.
The phase III Controlled Myelofibrosis Study with Oral JAK Inhibitor-I (COMFORT-I) and COMFORT-II trials showed significant benefits by reducing spleen size and relieving debilitating symptoms.
...
Wikipedia
