Irinotecan
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Camptosar (US), Campto (EU), Onivyde (liposomal) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608043 |
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Metabolism | Liver glucuronidation |
| Biological half-life | 6 to 12 hours |
| Excretion | Biliary and kidney |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.127.784 |
| Chemical and physical data | |
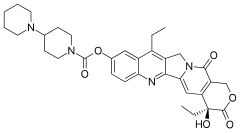
| Formula | C33H38N4O6 |
| Molar mass | 586.678 g/mol (irinotecan) 623.139 g/mol (irinotecan hydrochloride) 677.185 g/mol (irinotecan hydrochloride trihydrate) |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Irinotecan, sold under the brand name Camptosar among others, is a medication used to treat colon cancer and small cell lung cancer. For colon cancer it is used either alone or with fluorouracil. For small cell lung cancer it is used with cisplatin. It is given by slow injection into a vein.
Common side effects include diarrhea, vomiting, bone marrow suppression, hair loss, shortness of breath, and fever. Other severe side effects include blood clots, colon inflammation, and allergic reactions. Those with two copies of the UGT1A1*28 gene variant are at higher risk for side effects. Use during pregnancy can result in harm to the baby. Irinotecan is in topoisomerase inhibitor family of medication. It works by blocking topoisomerase 1 which results in DNA damage and cell death.
Irinotecan was approved for medical use in the United States in 1996. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. In the United Kingdom it is available as a generic medication and costs the NHS about 114.00 pounds per 100 mg. It is made from the natural compound camptothecin.
...
Wikipedia
