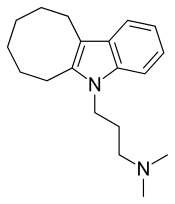
Iprindole
 |
|
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Biological half-life | 52.5 hours |
| Excretion | Urine, Feces |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.024.485 |
| Chemical and physical data | |
| Formula | C19H28N2 |
| Molar mass | 284.439 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Iprindole (Prondol, Galatur, Tertran), formerly known as pramindole, is a tricyclic antidepressant (TCA) used in Europe for the treatment of depression. It was introduced by Wyeth and has been used clinically since 1967. Notably, iprindole was the first second-generation antidepressant to be launched.
Iprindole is unique compared to most other TCAs in that it is a relatively weak inhibitor of the reuptake of serotonin and norepinephrine and instead acts predominantly as an antagonist of 5-HT2 receptors, hence its classification as 'second-generation'. Additionally, side effects of iprindole are much less prominent relative to other TCAs and it is well tolerated. However, iprindole's efficacy may not be as great as other TCAs, especially in regards to anxiety relief.
Iprindole has been sold under the trade name Prondol by Wyeth in the United Kingdom and Ireland for the indication of major depressive disorder, and has also been sold as Galatur and Tertran by Wyeth as well.
On a structural level, iprindole differs from other TCAs in that it contains an indole nucleus, similarly to the heterocyclic antipsychotic oxypertine, and has an eight-membered and saturated third ring.
...
Wikipedia
